* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download No Slide Title
Prescription costs wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Pharmacogenomics wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
NMDA receptor wikipedia , lookup
Psychedelic therapy wikipedia , lookup
Drug interaction wikipedia , lookup
Serotonin syndrome wikipedia , lookup
Cannabinoid receptor antagonist wikipedia , lookup
Urban legends about drugs wikipedia , lookup
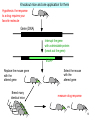
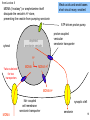
Polysubstance dependence wikipedia , lookup
Nicotinic agonist wikipedia , lookup
Amphetamine wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
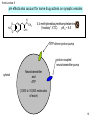
H 3C O O CH 3 N N N N CH 3 caffeine Bi 1 Lecture 13 Monday, April 24, 2005 revised 4/27 Recreational drugs 1 Disclaimer 1. Do not alter your pattern of prescription drug compliance as a result of this course. 2. Consult a medical professional for further guidance about prescription drugs. H. A. L. is not aware of all trends in current medical practice, is not a physician, and cannot prescribe. 2 CH 3 HO OH O N CH 3 HO morphine O H 3C N N O H3C H 3C H 3C O C5H11 H2 C OH ethanol tetrahydrocannabinol C2H5 O N C C2H5 CH 3 N N CH 3 N N CH 3 N phencyclidine caffeine LSD N CH 3 N H3C O OC C O nicotine O CH 3 N NH 2 CH 3 amphetamine cocaine 3 Coca Harvest in Bolivia, ca. 1950 4 cocaine in the test tube cocaine base: directly extracted from the plant with organic solvents H3C O OC C O CH 3 N cocaine hydrochloride: a salt, readily soluble treatment with HCl H+ O H3C O OC C O CH3 + HN Cl - O “crack” cocaine treatment with base: ammonia or Na bicarbonate, then heat to drive off HCl 5 cocaine in the body H3C O OC blood C O CH 3 N H3C O OC H+ O C O CH3 + HN O Lipid barrier, e. g. membrane(s) lungs, nose, stomach H3C O OC C O O cocaine base (crack) CH 3 N cocaine hydrochloride H+ H3C O OC C O CH3 HN + Cl- O South American Indians use Ca(OH)2 from limestone to shift this equilibrium 6 Targets for Recreational Drugs amphetamine(*) phencyclidine cocaine nicotine neurotransmitter transporters ligand-activated channels GPCRs enzymes N C LSD a G protein-activated channels morphine-heroin tetrahydrocannabinol caffeine* ?alcohol? (*= intracellular target) 7 From Lecture 5 Na+-coupled cell membrane neurotransmitter transporters: major targets for drugs of therapy and abuse Antidepressants (“SSRIs” = serotonin-selective reuptake inhibitors): Prozac, Zoloft, Paxil, Celexa, Luvox Drugs of abuse: MDMA Na+-coupled cell membrane serotonin transporter Attention-deficit disorder medications: Trademarks: Ritalin, Dexedrine, Adderall Presynaptic terminals Drugs of abuse: cocaine amphetamine Na+-coupled cell membrane dopamine transporter cytosol NH 3+ HO outside HO N H HO H2 C C H2 NH3+ 8 enzyme inhibitors neurotransmitter transport inhibitors 9 Endogenous ligand morphine-heroin agonist endorphins (peptides) THC agonist anandamide nicotine agonist acetylcholine cocaine antagonist dopamine amphetamine “false substrate”, antagonist noradrenaline, serotonin, dopamine ethanol agonist ?G protein? LSD agonist serotonin caffeine inhibitor cyclic AMP (intracellular) phencyclidine antagonist glutamate 11 Primary Target Details (dates: ) morphine-heroin GPCR (G protein-coupled receptor) (Gi) m-opioid receptor 1985-1993 THC GPCR (Gi) cannabinoid receptor 1988 nicotine agonist-activated channel a4b2 nicotinic acetylcholine receptor 1905-1995 cocaine cell membrane neurotransmitter transporter dopamine transporter 1980-1991 amphetamine vesicular & cell membrane neurotransmitter transporter vesicular monoamine transporter 1990 - 1995 ethanol ? K channel ? G protein-gated inward rectifier GIRK1/2 1993 - 1999 LSD GPCR (Gq) 5-HT2a receptor 1985-1990 caffeine enzyme cyclic AMP phosphodiesterase 1965 phencyclidine ligand-activated channel NMDA glutamate receptor 1965 12 Knockout mice and one application for them Hypothesis: the response to a drug requires your favorite molecule Gene (DNA) Interrupt the gene with a detectable protein (knock out the gene) EGFP Replace the mouse gene with the altered gene Breed many identical mice Select the mouse with the altered gene measure drug response vs 13 Knockout mice in Drugs and the Brain (Behavioral observations) 1. The m-opioid receptor m-opioid receptor KOs specifically lack responses to certain types of pain (next slide). 2. The a4b2 nicotinic receptor a4 or b2 nicotinic receptor knockouts: (1) respond less to nicotine in pain tests (next slide) (2) fail to self-administer nicotine (next slide). 3. The dopamine transporter Dopamine transporter knockout mice: (1) are hyperactive, (2) show less response to cocaine, (3) self-administer cocaine less 4. Cannabinoid receptors Cannabinoid receptor knockouts have little overt differences to normal mice. They don’t show these effects of THC and anandamide: (1) decreased pain responses and (2) decreased heart rate. --------------------------------------------------5. But NMDA receptor knockouts die at birth: an uninformative result 14 Two behavioral tests often used on knockout mice Pain: Mice are placed on a hotplate at 55o C. The experimenter notes the time to lick paws, jump, etc. The experiment terminates at 30 s, regardless of the outcome. A pain-relieving drug increases the time to react No permanent harm to the mouse . . . Carefully regulated: http://www.olar.caltech.edu/iacuc-sops.htm Self-administration of a drug 15 Source (species: ) “poppy that brings sleep” (opium) morphine-heroin Papaver somniferum tetrahydrocannabinol Cannabis sativa marijuana, hemp nicotine Nicotiana tabacum tobacco cocaine Erythroxylum coca coca synthetic amphetamine ethanol yeast LSD synthetic caffeine phencyclidine Coffea sp., Camellia sinensis Based on plant Saccharomyces cerevisiae (fermentation) ergot coffee tea wheat fungus; Salem witch trials? synthetic 16 Gordon A. Alles noted the properties of Ephedra vulgaris, used against asthma. He synthesized amphetamine (Benzedrine). Caltech BS, 1922; MS, 1924; PhD, 1926. Research Associate in Biology, 19391963 17 from Lecture 9 pH effects also account for some drug actions on synaptic vesicles O H N O CH3 H2C CH3 3,4-methylenedioxymethamphetamine (“ecstasy”, XTC) pKa ~ 8.5 ATP-driven proton pump proton-coupled neurotransmitter pump cytosol Neurotransmitter and ATP (1,000 to 10,000 molecules of each) 18 from Lecture 9 MDMA (“ecstasy”) or amphetamine itself dissipate the vesicle’s H+ store, preventing the vesicle from pumping serotonin Weak acids and weak bases short-circuit many vesicles! ATP-driven proton pump cytosol proton-coupled vesicular serotonin transporter depleted serotonin vesicle serotonin vesicle H+ “false substrate” for two transporters MDMA MDMA-H+ MDMA-H+ Na+-coupled cell membrane serotonin transporter MDMA synaptic cleft serotonin 19 The adjacent hydroxyl groups of dopamine are readily oxidized (may partially cause the degeneration of dopaminergic neurons in Parkinson’s disease) HO H2 C C H2 NH3+ HO . . . but we can use the fact to detect dopamine in real time in the brain: A 20th-Century battery from Chem 1 textbook (OGN) Figure 12-06 20 A modified patch clamp circuit and pipette allow us to detect dopamine electrochemically A cytosol synaptic cleft carbon fiber HO HO H2 C C H2 NH3+ 21 In dopamine transporter knockout mice (“DAT -/-”), presynaptic stimuli (“^”) lead to longer individual dopamine release pulses; but amphetamine fails to release dopamine Amphetamine Jones et al J Neurosci 18, p 1979 22 Routes into the body Eat Inhale Smoke Inject morphine-heroin tetrahydrocannabinol nicotine chew cocaine amphetamine ethanol LSD caffeine phencyclidine 23 Neurons that make dopamine: “pleasure-reward” system highlighted. Most drugs of abuse affect this system Nestler Figure 8-6 24 Most recreational drugs act at < 10-5 M. Ethanol is an exception Active Concentration morphine-heroin ~ 1 mM tetrahydrocannabinol ~ 1 mM nicotine ~ 1 mM cocaine ~ 1 mM amphetamine ~ 1 mM ethanol LSD > 1 mM ~ 10 nM ~ 10 mM caffeine phencyclidine ~ 1 mM 25 System-level Action Dopamine “Pleasure” system morphine-heroin Noradrenaline “Readiness” system “PerceptionAssociation” system “Decreased neuronal activity” tetrahydrocannabinol nicotine cocaine amphetamine ethanol LSD caffeine phencyclidine 26 Only a few thousand neurons in the brain make noradrenaline Nestler Figure 8-5 27 System-level Action Dopamine “Pleasure” system morphine-heroin Noradrenaline “Readiness” system “PerceptionAssociation” system “Decreased neuronal activity” tetrahydrocannabinol nicotine cocaine amphetamine ethanol ? LSD caffeine phencyclidine 28 Neurons that make serotonin project to many higher brain regions raphe nuclei simplified from Nestler Figure 9-3 29 Recreational drugs have varying overall effects Overall Action morphine-heroin inhibitory tetrahydrocannabinol inhibitory nicotine excitatory cocaine excitatory amphetamine excitatory ethanol inhibitory LSD caffeine phencyclidine hallucinations excitatory hallucinations 30 Legal status of Recreational Drugs Recreational use Prescription use morphine-heroin Illegal Schedule II pain control tetrahydrocannabinol Illegal; see next occasional nicotine Taxed; no sales to minors cocaine Illegal ENT surgery amphetamine Illegal Schedule II ADD / ADHD Diet pills ethanol Taxed; no sales to minors LSD Illegal With antihistamines Non-prescription use Smoking cessation: gum, patch caffeine Legal In some migraine medications phencyclidine Illegal none current 31 More on marijuana: legal status April 20, 2006 “US Inter-Agency Advisory Regarding Claims That Smoked Marijuana Is a Medicine” http://www.fda.gov/bbs/topics/NEWS/2006/NEW01362.html New York Times editorial page response 4/22/2006: “The Politics of Pot” “The Bush administration's habit of politicizing its scientific agencies was on display again this week when the Food and Drug Administration, for no compelling reason, unexpectedly issued a brief, poorly documented statement disputing the therapeutic value of marijuana. The statement was described as a response to numerous inquiries from Capitol Hill, but its likely intent was to buttress a crackdown on people who smoke marijuana for medical purposes and to counteract state efforts to legalize the practice. “Ordinarily, when the F.D.A. addresses a thorny issue, it convenes a panel of experts who wade through the latest evidence and then render an opinion as to whether a substance is safe and effective to use. This time the agency simply issued a skimpy one-page statement asserting that "no sound scientific studies" supported the medical use of marijuana. “That assertion is based on an evaluation by federal agencies in 2001 that justified the government's decision to tightly regulate marijuana under the Controlled Substances Act. But it appears to flout the spirit of a 1999 report from the Institute of Medicine, a unit of the National Academy of Sciences. “The institute was appropriately cautious in its endorsement of marijuana. It said the active ingredients of marijuana appeared useful for treating pain, nausea and the severe weight loss associated with AIDS. It warned that these potential benefits were undermined by inhaling smoke that is more toxic than tobacco smoke. So marijuana smoking should be limited, it said, to those who are terminally ill or don't respond to other therapies. “Yet the F.D.A. statement, which was drafted with the help of other federal agencies that focus on drug abuse, does not allow even that much leeway. It argues that state laws permitting the smoking of marijuana with a doctor's recommendation are inconsistent with ensuring that all medications undergo rigorous scrutiny in the drug approval process. “That seems disingenuous. The government is actively discouraging relevant research, according to scientists quoted by Gardiner Harris in yesterday's Times. It's obviously easier and safer to issue a brief, dismissive statement than to back research that might undermine the administration's inflexible 32 opposition to the medical use of marijuana.” H 3C O O CH 3 N N N N CH 3 caffeine End of Lecture 13 33