* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Stimulants - Littleton High School
Survey
Document related concepts
Transcript
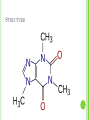
STIMULANTS By: Sloane Smith and Mariah White IB Chemistry AMPHETAMINES DEVELOPMENT Amphetamine was first synthesized by Lazar Edeleano in Germany in 1887, but it only entered clinical medicine in the late 1920s when its psychostimulant effect was recognized. Amphetamine is structurally related to ephedrine, a natural stimulant found in plants of the genus Ephedra that was used to dilate the bronchial sacs of the lungs in the treatment of breathing disorders. The US medical and pharmaceutical establishment was worried that supplies of Ephedra in faraway China would be exhausted so an alternative was synthesized. Amphetamine promised a cheap and synthetic substitute. EPINEPHRINE VS. AMPHETAMINE Amphetamine is structurally related to epinephrine (derived from the phenylethylamine structure)and therefore produces similar results. Sympathomimetic drugs (substances that mimic the effects of the sympathetic [fight or flight] nervous system) Amphetamine: http://upload.wikimedia.org/wikipedia/commons/6/68/ Amphetamine_structure.png Nonpolar Epinephrine: http://upload.wikimedia.org/wikipedia/commons/thum b/2/2f/Epinephrine_structure.svg/426pxEpinephrine_structure.svg.png Polar HOW DO THEY WORK When ingested, many of the biological reactions normally controlled by neurotransmitters are elicited. Because it is not metabolized as rapidly as adrenaline, noradrenaline, and dopamine (these are all polar where amphetamine is nonpolar), it remains active in the body longer and effects can still be felt four to six hours after oral ingestion of a relatively small dose. Use results in an increase the synaptic activity of the dopamine and norepinephrine neurotransmitter systems causing: the release of dopamine from axon terminals blocking dopamine reuptake Inhibition of the storage of dopamine in vesicles Inhibition of the destruction of dopamine by enzymes PHYSIOLOGICAL EFFECTS The drug results in a increased blood pressure, heart rate, and body temperature, dilated pupils, decreased appetite and fatigue, and stimulated respiration, followed by feelings of happiness and power . Using 10-15 mg daily allows an individual to feel alert and confident in performing physical and mental work providing for an increase in levels of activity. USES Commonly used to treat ADHD in adults and children, asthma symptoms of traumatic brain injury, and daytime drowsiness symptoms of narcolepsy and chronic fatigue syndrome. Initially it was popular to diminish the appetite and to control weight. EXAMPLES Dexedrine, Benzedrine, Ritalin, Adderall, Vyvanse, Methamphetamine , and Ecstasy. DEPENDENCY Dependency arises from the desire to continue and heighten the euphoric effects of the drug. Long terms effects include: insomnia, restlessness, “paranoid psychosis”, hallucinations, violent and aggressive behavior, weight loss, and tremors INTERESTING FACTS From 1942, Hitler received daily methamphetamine injections During World War II amphetamine was extensively used to combat fatigue and increase alertness in soldiers Under Canada's Controlled Drugs and Substances Act, possession of amphetamines or their derivatives is a criminal offense CAFFEINE GENERAL INFORMATION Caffeine = Trimethylxanthine (C8H10N4O2) Found in coffee beans, tea leaves, cacao beans, some soft drinks, energy drinks, and also some pain killers and other medicine. Caffeine is addictive. Alkaloid: Compounds from plants. They all have nitrogen, a tertiary amine, and heterocyclic rings. Other examples: morphine, codeine, cocaine, and nicotine. USES Medical Uses: Mild diuretic Cardiac Stimulant Recreational Uses: Increases alertness Increases concentration (Helps people to stay awake…) HOW DOES CAFFEINE WORK? Adenosine (a chemical found in the brain) bonds to adenosine receptors. 1) • Caffeine because it is so structurally similar to adenosine, is able to bind to adenosine receptors, thus blocking adenosine. 2) • • 3) Slows nerve cell activity and causes blood vessels to dilate Nerve cells speed up Neuron firing increases Pituitary glands tricked into producing adrenaline STRUCTURE EFFECTS Increases dopamine levels (like amphetamines, heroine, and cocain) Dopamine: Neurotransmitter . Activates “pleasure center.” Linked to causes for addiction Sort-term Effects: Increases alertness, concentration, and restlessness Long-term Effects: Increased irritability and jumpiness Sleep problems Caffeine’s half-life is about 6 hrs. (If 200mg is consumed at 3:00pm, 100mg will still be there at 9pm.) Increases dependency ADDICTION… “When you get in the cycle of using caffeine, you have to keep taking the drug. Even worse, if you try to stop taking caffeine, you get very tired and depressed and you get a terrible, splitting headache as blood vessels in the brain dilate. These negative effects force you to run back to caffeine even if you want to stop.” -Marshall Brain, How Stuff Works INTERESTING INFORMATION Theobromine in chocolate, a chemical that is similar to caffeine, is toxic to dogs (100-150 mg/kg of body weight) This can occur in humans too. CHOCOLATE POISONING 150 mg/kg Children and infants should stay away from caffeine… Recent studies have shown…: “Regular coffee drinkers are 80 percent less likely to develop Parkinson's disease. Two cups a day gives you 20 percent less risk of colon cancer. Two cups a day causes an 80 percent drop in cirrhosis. Two cups a day prevents gallstone development by 50 percent. It has also shown to be beneficial in asthma, stopping headaches, boosting mood and even preventing cavities” (howitworks.com) NICOTINE GENERAL INFORMATION C10H14N2 Found in tobacco plant Sympathomimetic Drug that mimics the effects of the sympathetic nervous system (speeds heart, contracts blood vessels) Increases concentration Releases tension Absorbed through: Skin Lungs Mucous membranes STRUCTURE HOW IT WORKS Once absorbed, Nicotine travels through the blood stream to the brain. This then releases it to the rest of your body. This happens quickly (10-15 minutes) Removal Process: Enzymes in liver break about 80% down into cotinine. Also metabolized in lungs to cotinine and nicotine oxide. Cotinine is excreted through urine. Kidneys filter all remaining nicotine which is then also excreted through urine. HOW IT WORKS (CONTINUED) Acetylcholine: Neurotransmitter. Delivers signals from brain to muscles Controls functions such as heart beat, breathing, and energy level Directs flow of information to brain When involving nicotine…: Neurons release more acetylcholine Activates “reward pathways” Heightened cholinergic activity (Increases awareness) Gives you pleasant emotions → leads to addiction Glutamate (neurotransmitter involved in memory and learning) released Can create a “memory loop” of pleasant emotions HOW IT WORKS (CONTINUED) USES Studies have shown that nicotine may slow an on-set of Alzheimer’s Disease Cholinergic Nerves May reduce symptoms of Turrette's Syndrome PHYSICAL EFFECTS Short-term: Can relax and/or invigorate Causes release of adrenaline Long-term: May block insulin release May increase basal metabolic rate Burn more calories at rest HOWEVER, this may also increase LDL levels in the body. Higher risk of heart attack or stroke. Increased risk of heart problems (disease), coronary thrombosis, Emphysema, Cancer, and Stroke Increase of stomach acid Increased risk of peptic ulcers SMOKING There are 8 to 20mg of Nicotine per cigarette Only 1mg is actually absorbed… Risks: Chronic lung disease, cancers of the lung, mouth, and throat, and it can cause problems in pregnancies. Withdrawal Symptoms: Nausea, weight gain, insomnia, irritability, and depression INTERESTING INFORMATION 5% of the tobacco plant is nicotine. There is a genetic defect in which the liver cannot process and break down nicotine. This causes nicotine levels to stay higher for longer periods of time without smoking. Nicotine increases the number of neurotransmitters and chemicals in the brain Endorphins WORKS CITED Brain, M. (2008). How Stuff works. Hoboken, NJ: Cartwell Books, Inc. http://faculty.washington.edu/chudler/amp.html http://www.aadac.com/87_419.asp www.un.org/ga/20special/featur/amphet.htm http://www.well.com/user/woa/fsstim.htm http://medicaldictionary.thefreedictionary.com/sympathomimet ic http://health.howstuffworks.com/caffeine.htm http://health.howstuffworks.com/caffeine.htm http://www.natural-remedies-review.com/imagefiles/chemical-structure-caffeine.png