* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Introduction to Atomic Structure
Density of states wikipedia , lookup
Casimir effect wikipedia , lookup
Quantum vacuum thruster wikipedia , lookup
Aharonov–Bohm effect wikipedia , lookup
Introduction to gauge theory wikipedia , lookup
Conservation of energy wikipedia , lookup
Nuclear physics wikipedia , lookup
Photon polarization wikipedia , lookup
Old quantum theory wikipedia , lookup
Bohr–Einstein debates wikipedia , lookup
Diffraction wikipedia , lookup
Thomas Young (scientist) wikipedia , lookup
Time in physics wikipedia , lookup
Electromagnetism wikipedia , lookup
Photoelectric effect wikipedia , lookup
Hydrogen atom wikipedia , lookup
Electromagnetic radiation wikipedia , lookup
Introduction to quantum mechanics wikipedia , lookup
Wave–particle duality wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
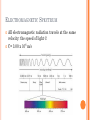
JOURNAL #37 What is the Bohr model? Refer to your textbook as needed. INTRODUCTION TO ATOMIC STRUCTURE TODAY’S LEARNING GOAL: We will describe the Bohr model. LIGHT AND SOUND In 1905 Einstein derived an equation relating mass and energy. You should be familiar with this equation: E = mc2 This equation has been changed a bit since, but a relationship has now, for the first time in history, been established between matter and energy and between physics and chemistry LIGHT AND SOUND Because Einstein was able to prove a relationship between matter and energy, we today can understand more about matter by learning all about energy. We can see this relationship between energy and matter specifically when we look at some of the unusual properties of the wave nature of energy THE NATURE OF LIGHT: WAVE OR PARTICLE? The nature of light has been debated for thousands of years. In the 1600’s Newton argued that light was a stream of particles. Huygens countered that it was a wave. Both had good arguments, but neither could prove it YOUNG’S DOUBLE SLIT EXPERIMENT In 1801, Thomas Young settled the argument with his Double Slit Experiment. We will take a closer look at the results of this experiment, but first we need to understand waves… http://video.mit.edu/watch/thomas-youngs-double-slit-experiment-8432/ YOUNG’S DOUBLE SLIT EXPERIMENT Young tested to see if light was a wave by seeing if it created an interference pattern when it went through the 2 slits, like a wave would. The double slit experiment relies on 2 properties of waves: Diffraction Interference Each slit generates a new wave due to diffraction. Those waves then either constructively or destructively interfere on a far away screen. LET’S REVIEW WHAT WE’VE LEARNED: What principle is responsible for light spreading as it passes through a narrow slit? A. Diffraction B. Polarization C. Dispersion D. interference Answer: A DOUBLE SLIT MAXIMA AND MINIMA Interference occurs because each point on the screen is not the same distance from both slits. Depending on the path length distance, the wave can interfere constructively or destructively Bright lines- Maxima Dark lines -Minima LET’S REVIEW WHAT WE’VE LEARNED: What principle is responsible for alternating light and dark bands when light passes through 2 or more narrow slits? A. Diffraction B. Polarization C. Dispersion D. interference Answer: D IF LIGHT IS A WAVE…WHAT IS WAVING? In sound waves, we know it’s the pressure in the air. In any simple harmonic motion there has to be 2 forms of energy and a means to move between them. But what does that mean for light? ACCELERATING CHARGES CREATE E-M WAVES A great way to start this up is to make a charge (like an electron) accelerate. That creates a changing electric field which creates a changing magnetic field Which creates a changing electric field… which creates a changing magnetic field Which creates a changing electric field… which creates a changing magnetic field Which creates a changing electric field… which creates a changing magnetic field CREATING ELECTROMAGNETIC WAVES In physics we learned that changing magnetic field produces an electric field. Changing an electric field produces a magnetic field as well Once these changing fields are first started up, they keep creating each other…and travel on their own. These traveling fields are called electromagnetic waves. LET’S REVIEW WHAT WE’VE LEARNED: An electric field is produced by a A. Constant magnetic field B. Changing magnetic field C. Either a constant or a changing magnetic field D. Gravitation Answer: B LET’S REVIEW WHAT WE’VE LEARNED: A changing electric field will produce a A. Current B. Gravitation field C. Magnetic field Answer: C LIGHT IS AN ELECTROMAGNETIC WAVE Young showed that light is a wave. Electromagnetic waves exist and travel at the speed of light Light was shown to be an electromagnetic wave The frequency of an electromagnetic wave is related to its wavelength. For electromagnetic waves, in a vacuum C=λV C = speed of light, λ = wavelength (m) V = frequency ELECTROMAGNETIC SPECTRUM All electromagnetic radiation travels at the same velocity: the speed of light © C= 3.00 x 108 m/s LET’S REVIEW WHAT WE’VE LEARNED: All electromagnetic waves travel through a vacuum at A. Same speed B. Speeds that are proportional to their frequency C. Speeds that are inversely proportional to their frequency D. Speeds too slow to measure Answer: A WHY DOES THIS ALL MATTER? Light behaves like a wave and so does matter! Electrons fired on at a time towards two slits show the same interference pattern when they land on a distant screen. Since all matter and energy are now understood they share certain properties (wavelength for example) the interaction of matter with light has allowed us to probe the nature of matter itself, from the structure of the atom to the unique behavior of molecules. The structure and behavior of matter is the domain of the chemist!! QUANTUM A quantum of energy is the minimum quantity of energy that can be lost or gained by an atom The relationship between a quantum of energy and the frequency of radiation is E=hv E= energy (joules), V= frequency and h= is a funamental physical constant (planck’s constant) 6.626 x 10-34J.s THE HYDROGEN ATOM LINE EMISSION SPECTRUM When current is passed through a gas at low pressure, the potential energy of some of the gas atoms increases. The lowest energy state of an atom is its ground state. A state in which an atom has a higher potential energy than it has in its ground stat is an excited state. When an excited atom returns to its ground state, it gives off the energy it gained in the form of electromagnetic radiation. (ex: neon lights) THE HYDROGEN ATOM LINE EMISSION SPECTRUM When a narrow beam of emitted light was shined through a prism, it was separated into four specific colors of the visible spectrum. The four bands of light were part of what is known as Hydrogen’s line-emission spectrum. Attempts to explain why hydrogen atoms gave off only specific frequencies of light is called quantum theory. THE HYDROGEN ATOM LINE EMISSION SPECTRUM When an excited Hydrogen atom falls to its ground state or to a lower energy excited state, it emits a photon of radiation. The energy of this photon (E=hv) is equal to the difference in energy between the atoms initial state and its final state. The fact that hydrogen atoms emit only specific frequencies of light indicated that the energy differences between the atom’s energy states were fixed. (hydrogen atoms exists only in very specific energy states) The BOHR MODEL puzzle of the hydrogen atom spectrum was solved by Niels Bohr. The electron can circle the nucleus only in allowed paths, or orbits. When the electron is in one of these orbits, the atom has a definite, fixed energy. The electron is in its lowest energy state when it is in the orbit closest to the nucleus. This orbit is separated from the nucleus by a large empty space where the electron cannot exist. The energy is higher when the electron is in orbits that are farther from the nucleus. BOHR MODEL Example: When standing on a ladder, the higher up you go, the more potential energy you have. Your energy cannot correspond to standing between 2 steps because you cannot stand in midair. The same way for electrons. They can be in one orbit or another, but not in between. BOHR MODEL How does this explain the observed spectral lines? While in a given orbit, the electron is neither gaining nor losing energy However, it can move to a higher energy orbit by gaining an amount of energy equal to the difference in energy between the higher energy orbit and initial lower energy orbit. BOHR MODEL When a H atom is in excited state, its electron is in one of the higher energy level orbits. When the electron falls to a lower energy level, a photon is emitted – called emission. Absorption is the process in which energy must be added to an atom in order to move an electron from a lower energy level to a higher energy. YOUR ASSIGNMENT Chapter 4 section 1 review (pg 103) Do problems 1-5.