* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Alcohols
Survey
Document related concepts
Elias James Corey wikipedia , lookup
Asymmetric induction wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Hydroformylation wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Transcript
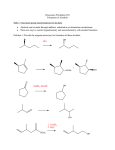
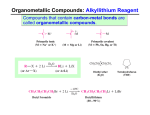
Organic Chemistry, 6th Edition L. G. Wade, Jr. Chapter 10 Structure and Synthesis of Alcohols Structure of Alcohols • Hydroxyl (-OH) functional group • Oxygen is sp3 hybridized. Chapter 10 2 Classification • Primary: carbon with –OH is bonded to one other carbon. • Secondary: carbon with –OH is bonded to two other carbons. • Tertiary: carbon with –OH is bonded to three other carbons. • Aromatic (phenol): –OH is bonded to a benzene ring. Chapter 10 3 IUPAC Nomenclature • Find the longest carbon chain containing the carbon with the -OH group. • Drop the -e from the alkane name, add -ol. • Number the chain, starting from the end closest to the -OH group. • Number and name all substituents. Chapter 10 4 Unsaturated Alcohols • Hydroxyl group takes precedence. Assign that carbon the lowest number. • Use alkene or alkyne name. 4-penten-2-ol pent-4-ene-2-ol Chapter 10 5 Naming Priority • • • • • • Acids Esters Aldehydes Ketones Alcohols Amines • • • • • Chapter 10 Alkenes Alkynes Alkanes Ethers Halides 6 Hydroxy Substituent • When -OH is part of a higher priority class of compound, it is named as hydroxy. • Example: 4-hydroxybutanoic acid Chapter 10 7 Common Names • Alcohol can be named as alkyl alcohol. • Useful only for small alkyl groups. • Examples: isobutyl alcohol sec-butyl alcohol => Chapter 10 8 Naming Diols • Two numbers are needed to locate the two -OH groups. • Use -diol as suffix instead of -ol. hexane-1,6- diol Chapter 10 9 Glycols • 1, 2 diols (vicinal diols) are called glycols. • Common names for glycols use the name of the alkene from which they were made. ethane-1,2- diol propane-1,2- diol ethylene glycol propylene glycol Chapter 10 10 Naming Phenols • -OH group is assumed to be on carbon 1. • For common names of disubstituted phenols, use ortho- for 1,2; meta- for 1,3; and para- for 1,4. • Methyl phenols are cresols. 4-methylphenol para-cresol 3-chlorophenol meta-chlorophenol Chapter 10 11 Physical Properties • Unusually high boiling points due to hydrogen bonding between molecules. • Small alcohols are miscible in water, but solubility decreases as the size of the alkyl group increases. Chapter 10 12 Boiling Points => Chapter 10 13 Solubility in Water Solubility decreases as the size of the alkyl group increases. Chapter 10 14 Methanol • • • • “Wood alcohol” Industrial production from synthesis gas Common industrial solvent Fuel at Indianapolis 500 Fire can be extinguished with water High octane rating Low emissions But, lower energy content Invisible flame Chapter 10 15 Ethanol • • • • • • • Fermentation of sugar and starches in grains 12-15% alcohol, then yeast cells die Distillation produces “hard” liquors Azeotrope: 95% ethanol, constant boiling Denatured alcohol used as solvent Gasahol: 10% ethanol in gasoline Toxic dose: 200 mL ethanol, 100 mL methanol Chapter 10 16 2-Propanol • “Rubbing alcohol” • Catalytic hydration of propene Chapter 10 17 Acidity of Alcohols • pKa range: 15.5-18.0 (water: 15.7) • Acidity decreases as alkyl group increases. • Halogens increase the acidity. • Phenol is 100 million times more acidic than cyclohexanol! Chapter 10 18 Table of Ka Values => Chapter 10 19 Formation of Alkoxide Ions React methanol and ethanol with sodium metal (redox reaction). React less acidic alcohols with more reactive potassium. Chapter 10 20 Formation of Phenoxide Ion Phenol reacts with hydroxide ions to form phenoxide ions - no redox is necessary. O O H + OH + HOH pKa = 15.7 pKa = 10.0 Chapter 10 21 Synthesis (Review) • Nucleophilic substitution of OH- on alkyl halide • Hydration of alkenes water in acid solution (not very effective) oxymercuration - demercuration hydroboration - oxidation Chapter 10 22 Organometallic Reagents • Carbon is bonded to a metal (Mg or Li). • Carbon is nucleophilic (partially negative). • It will attack a partially positive carbon. C - X C = O • A new carbon-carbon bond forms. Chapter 10 23 Grignard Reagents • • • • Formula R-Mg-X (reacts like R:- +MgX) Stabilized by anhydrous ether Iodides most reactive May be formed from any halide primary secondary tertiary vinyl aryl Chapter 10 24 Some Grignard Reagents Chapter 10 25 Organolithium Reagents • Formula R-Li (reacts like R:- +Li) • Can be produced from alkyl, vinyl, or aryl halides, just like Grignard reagents. • Ether not necessary, wide variety of solvents can be used. Chapter 10 26 Reaction with Carbonyl • R:- attacks the partially positive carbon in the carbonyl. • The intermediate is an alkoxide ion. • Addition of water or dilute acid protonates the alkoxide to produce an alcohol. Chapter 10 27 Synthesis of 1° Alcohols Grignard + formaldehyde yields a primary alcohol with one additional carbon. Chapter 10 28 Synthesis of 2º Alcohols Grignard + aldehyde yields a secondary alcohol. Chapter 10 29 Synthesis of 3º Alcohols Grignard + ketone yields a tertiary alcohol. Chapter 10 30 Grignard Reactions with Acid Chlorides and Esters • Use two moles of Grignard reagent. • The product is a tertiary alcohol with two identical alkyl groups. • Reaction with one mole of Grignard reagent produces a ketone intermediate, which reacts with the second mole of Grignard reagent. => Chapter 10 31 Grignard + Acid Chloride (1) • Grignard attacks the carbonyl. • Chloride ion leaves. Chapter 10 32 Grignard and Ester • Grignard attacks the carbonyl. • Alkoxide ion leaves! ? ! Chapter 10 33 Second step of reaction • Second mole of Grignard reacts with the ketone intermediate to form an alkoxide ion. • Alkoxide ion is protonated with dilute acid. Chapter 10 34 Limitations of Grignard • No water or other acidic protons like O-H, N-H, S-H, or -C—C-H. Grignard reagent is destroyed, becomes an alkane. • No other electrophilic multiple bonds, like C=N, CN, S=O, or N=O. Chapter 10 35 Reduction of Carbonyl • Reduction of aldehyde yields 1º alcohol. • Reduction of ketone yields 2º alcohol. • Reagents: Sodium borohydride, NaBH4 Lithium aluminum hydride, LiAlH4 Raney nickel Chapter 10 36 Sodium Borohydride • Hydride ion, H-, attacks the carbonyl carbon, forming an alkoxide ion. • Then the alkoxide ion is protonated by dilute acid. • Only reacts with carbonyl of aldehyde or ketone, not with carbonyls of esters or carboxylic acids. Chapter 10 37 Lithium Aluminum Hydride • Stronger reducing agent than sodium borohydride, but dangerous to work with. • Converts esters and acids to 1º alcohols. Chapter 10 38 Comparison of Reducing Agents • LiAlH4 is stronger. • LiAlH4 reduces more stable compounds which are resistant to reduction. => Chapter 10 39 Catalytic Hydrogenation • Add H2 with Raney nickel catalyst. • Also reduces any C=C bonds. Chapter 10 40 End of Chapter 10 Chapter 10 41