* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Organic Chem Functional Groups
Homoaromaticity wikipedia , lookup
Kinetic resolution wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Petasis reaction wikipedia , lookup
Asymmetric induction wikipedia , lookup
Organosulfur compounds wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
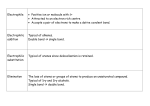
Chapter 22 “Functional Groups” Functional Groups Most organic chemistry involves substituents often contain O, N, S, or P also called “functional groups”- they are the chemically functional part of the molecule, and are the non-hydrocarbon part Functional Groups Functional group - a specific arrangement of atoms in an organic compound, that is capable of characteristic chemical reactions. What is the best way to classify organic compounds? By their functional groups. Functional Groups The symbol “R” is used to represent any carbon chains or rings Important: Table 22.2, page 589 shows some of the major functional groups - KNOW THESE. Halogen Substituents Halocarbons - class of organic compounds containing covalently bonded fluorine, chlorine, bromine, or iodine General formula: R-X (X = halogen) Naming? Name parent as normal, add the halogen as a substituent (or prefix) Halogen Substituents The more highly halogenated the compound is, the higher the b.p. Few halocarbons found in nature but, readily prepared and used halothane and also the hydrofluorocarbons Substitution Reactions Organic reactions often much slower than inorganic reactions must break strong covalent bond trying to find new catalysts to use Substitution - an atom (or group of atoms) replaces another atom or group of atoms Substitution Reactions A halogen (shown as “X”) can replace a hydrogen to make a halocarbon: R-H + X2 R-X + HX Sunlight is often a sufficient catalyst: CH4 + Cl2 UV light → CH3Cl + HCl Substitution Reactions Treating benzene with a halogen? Examples on Page 601 C6H6(l) + Cl2(g) → C6H5Cl(l) + HCl (g) Halogens on carbon chains are readily displaced by hydroxide ions (OH1-) to make an alcohol + a salt: R-X + OH1- R-OH + X1CH3-Cl + NaOH CH3-OH + NaCl Methanol + sodium chloride Substitution Reactions CH3-I + KOH CH3-OH + KI Iodomethane Methanol CH3CH2Br + NaOH CH3CH2OH + NaBr Bromoethane Ethanol Alcohols Alcohols - a class of organic compounds with an -OH group The -OH functional group in alcohols is called a “hydroxyl” group; thus R-OH is the formula How is this different from the hydroxide ion? (covalent bonding with the carbon- not ionic with a metal like bases) Alcohols Aliphatic alcohols classified into categories according to the number of R groups attached to the carbon with the hydroxyl 1 R group: primary alcohol 2 R groups: secondary alcohol 3 R groups: tertiary alcohol Alcohols Both IUPAC and common names For IUPAC: drop the -e ending of the parent alkane name; add ending of -ol, number the position of -OH parent is the longest chain that contains the carbon with the hydroxyl attached. Alcohols The hydroxyl is given the lowest position number Alcohols containing 2, 3, and 4 of the -OH substituents are named diols, triols, and tetrols respectively Alcohols Common names: similar to halocarbons, meaning name the alkyl group, then followed by the word alcohol One carbon alcohol = methyl alcohol Alcohols More than one -OH substituents are called glycols (ethylene glycol?) ** Examples on page 590** Phenols - compounds in which a hydroxyl group is attached directly to an aromatic ring. Cresol is the common name of o, m, and p isomers of methylphenol Properties of Alcohols Much like water, alcohols are capable of hydrogen bonding between molecules this means they will boil at a higher temp. than alkanes and halocarbons with a comparable number of atoms Properties of Alcohols Alcohols are derivates of water; the -OH comes from water, and thus are somewhat soluble Alcohols of up to 4 carbons are soluble in water in all proportions; more than 4 carbons are usually less soluble, because the longer carbon chain is more nonpolar Properties of Alcohols Many aliphatic alcohols used in laboratories, clinics, and industry Isopropyl alcohol (2propanol) is rubbing alcohol; used as antiseptic, and a base for perfume, creams, lotions, and other cosmetics Ethylene glycol (1,2-ethanediol) commonly sold as “antifreeze” Properties of Alcohols Glycerol (1,2,3-propanetriol) used as a moistening agent in cosmetics, foods, and drugs; also a component of fats and oils Ethyl alcohol (ethanol) used in the intoxicating beverages; also an important industrial solvent Properties of Alcohols Denatured alcohol- means it has been made poisonous by the addition of other chemicals, often methyl alcohol (methanol, or wood alcohol). As little as 10 mL of methanol has been known to cause permanent blindness, and 30 ml has resulted in death! Addition Reactions The carbon-carbon single bond is not easy to break In double bonded alkenes, it is easier to break a bond Addition reaction- substance is added at the double or triple bond location, after it is broken Addition Reactions Addition of water to an alkene is a hydration reaction usually occurs with heat and an acid (such as HCl or H2SO4 acting as a catalyst) Note sample in the middle of page 590 for the formation of ethanol from ethene + water Addition Reactions Addition of hydrogen to produce an alkane is a hydrogenation reaction, which usually involves a catalyst such as Pt or Pd common application is the manufacture of margarine from unsaturated vegetable oils (making them solid from a liquid) Ethers A class of organic compounds in which oxygen is bonded to 2 carbon groups: R-O-R is formula Naming? The two R groups are alphabetized, and followed by ether Two R groups the same? Use the prefix di- Ethers Diethyl ether is the one commonly called just “ether” was the first reliable general anesthetic dangerous- highly flammable, also causes nausea ethers are fairly soluble in water Aldehydes and Ketones Review: alcohol has an oxygen bonded to a carbon group and a hydrogen ether has an oxygen bonded to two carbon groups An oxygen can also be bonded to a single carbon by a double bond Aldehydes and Ketones The C=O group is called the “carbonyl group” it is the functional group in both aldehydes and ketones Aldehydes - carbonyl group always joined to at least one hydrogen (meaning it is always on the end!) Aldehydes and Ketones Ketones - the carbon of the carbonyl group is joined to two other carbons (meaning it is never on the end) Aldehydes and Ketones Naming? Aldehydes: identify longest chain containing the carbonyl group, then the -e ending replaced by -al, such as methanal, ethanal, etc. Ketones: longest chain w/carbonyl, then new ending of -one; number it? propanone, 2-pentanone, 3pentanone Aldehydes and Ketones Neither can form intermolecular hydrogen bonds, thus a much lower b.p. than corresponding alcohols wide variety have been isolated from plants and animals; possible fragrant odor or taste; many common names Aldehydes and Ketones Benzaldehyde Cinnamaldehyde Vanillin See page 592 for structures of these 3 Methanal (the common name is: formaldehyde) 40% in water is formalin, a preservative Aldehydes and Ketones Propanone (common: acetone) is a good solvent; miscible with water in all proportions why is it a good substance used in nail-polish removers? (a powerful solvent-able to dissolve both polar & nonpolar) The Carboxylic Acids… Also have a carbonyl group (C=O), but is also attached to a hydroxyl group (-OH) = “carboxyl” group general formula: R-COOH weak acids (ionize slightly) Named by replacing -e with -oic and followed by the word acid methanoic acid; ethanoic acid Carboxylic Acids Abundant and widely distributed in nature, many having a Greek or Latin word describing their origin acetic acid (ethanoic acid) from acetum, meaning vinegar many that were isolated from fats are called fatty acids The Esters… General formula: RCOOR Derivatives of the carboxylic acids, in which the -OH from the carboxyl group is replaced by an -OR from an alcohol: carboxylic acid + alcohol ester + water many esters have pleasant, fruity odors- banana, pineapple, perfumes Esters Although polar, they do not form hydrogen bonds (reason: there is no hydrogen bonded to a highly electronegative atom!) thus, much lower b.p. than the hydrogen-bonded carboxylic acids they came from Esters Can be prepared from a carboxylic acid and an alcohol; usually a trace of mineral acid added as catalyst (because acids are dehydrating agents) Note equation on p. 593 Esters Naming? It has 2 words: 1st: alkyl attached to single bonded oxygen from alcohol 2nd: take the acid name, remove the -ic acid, add -ate example on top of page 593 Amines Amines contain a basic nitrogen atom with a lone pair. Amines are derivatives of ammonia, where in one or more hydrogen atoms have been replaced by a substituent such as an alkyl group. Important amines include amino acids, biogenic amines, trimethylamine, and aniline Primary amine Secondary amine Tertiary amine Isomers Isomers are compounds with the same molecular formula but different structural formulas A simple example of isomerism is given by the formula C3H8O (or C3H7OH) and occurs as two isomers: 1 -propanol and 2- propanol Note that the position of the oxygen atom differs between the two: it is attached to an end carbon in the first isomer, and to the center carbon in the second. There is, however, another isomer of C3H8O which has significantly different properties: methyl-ethylether. Unlike the isomers of propanol, the ether has an oxygen connected to two carbons rather than to one carbon and one hydrogen. Geometric Isomers In one, the two chlorine atoms are locked on opposite sides of the double bond. This is known as the trans isomer. (trans : from latin meaning "across" - as in transatlantic). In the other, the two chlorine atoms are locked on the same side of the double bond. This is know as the cis isomer. (cis : from latin meaning "on this side" cis & trans isomers differ from each other in their physical properties and to a lesser extent their chemical properties. Optical Isomers (enantiomers) These two models each have the same groups joined to the central carbon atom, but still manage to be different: Obviously as they are drawn, the orange and blue groups aren't aligned the same way. Could you get them to align by rotating one of the molecules? The next diagram shows what happens if you rotate molecule B. They still aren't the same - and there is no way that you can rotate them so that they look exactly the same. These are isomers of each other. They are described as being non-superimposable in the sense that you couldn't slide one molecule exactly over the other one. Something would always be pointing in the wrong direction. These molecules are said to be chiral. Chiral molecules have the same physical properties, but b/c they differ in the 3-D structue they may show different physiological properties. (Aleve, Oxidation- Reduction Reactions All of the previous classes of organic compounds are related by oxidation and reduction reactions What is oxidation-reduction? Oxidation: the gain of oxygen, loss of hydrogen, or loss of e-1 Reduction: the loss of oxygen, gain of hydrogen, or gain of e-1 Oxidation- Reduction Reactions Oxidation and reduction reactions (sometimes called redox) are coupled- one does not occur without the other The number of Oxygen and Hydrogen attached to Carbon indicates the degree of oxidation Oxidation- Reduction Reactions The fewer the # of H on a C-C bond, the more oxidized the bond Thus, a triple bond is more oxidized than a double bond and a single bond An alkane is oxidized (loss of H) to an alkene, and then to an alkyne Oxidation- Reduction Reactions Loss of hydrogen is called a dehydrogenation reaction may require strong heating and a catalyst Oxidation- Reduction Reactions Methane can be oxidized in steps to carbon dioxide methane methanol methanal methanoic acid CO2 the more reduced (more H) a carbon compound, the more energy it can release upon oxidation