* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Alcohols
Survey
Document related concepts
Transcript
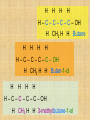
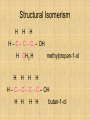
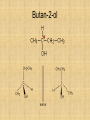
Isomers For each of the following: 1 2 3 Name the compounds shown. Identify the structural isomers. Identify the geometric isomers. butane methylpropane but-1-ene trans but-2-ene propene ethene methylpropene cis but-2-ene IUPAC name = Alkanols • CnH2n+1OH for saturated acohols • Can be written R-OH • Alcohols with three or more carbons form structural isomers. Alcohols Alcohols Naming and Structure • -ol replaces the –e • Parent chain contains –OH • Position of hydroxyl group determines numbering of alkyl groups (i.e count from the lowest carbon chain) • Prefix –di, -tri indicate multiple functional groups H H H H H – C – C – C – C – OH H CH3 H H Butane H H H H H – C – C – C – C – OH H CH3 H H Butan-1-ol H H H H H – C – C – C – C – OH H CH3 H H 3-methylbutane-1-ol Properties • Hydrogen bonding due to –OH groups creating polarity – Determines physical properties for smaller molecules • Permanent dipoles and instantaneous dipoles – Non-polar hydrocarbon portion determines properties for larger molecules pH & Conduction • Non-conductors • Neutral solutions Melting & Boiling Points • Higher than corresponding alkane due to hydrogen bonding • Liquids at room temp up to 8Carbons Solubility • Up to 3 Carbon soluble in very water • 4 Carbon soluble in water • 5-6 Carbon slightly soluble Solubility due to polarity • >6 C insoluble in water due to large nonpolar alkyl (hydrocarbon) region. • Commercial solvent as they can dissolve both polar and non-polar substances. 1,2-Ethanediol • Two hydroxyl groups = greater hydrogen bonding • Antifreeze • Antiboil • De-icing fluid Alcohols II Isomerism Cis-trans isomers 1 2 3 4 5 Which of the following compounds will have cis-trans isomers? (Hint: Draw the structure.) CHCl=CH2 The following have cis-trans isomers CH3CH=CHCH2CH3 2 CH3CH=CHCH2CH3 CHCl=CHCl 3 CHCl=CHCl CH2=CBr2 5 CHBr=CBrCH3 CHBr=CBrCH3 Structural Isomerism H H H H – C – C – C – OH H CH3 H H H H methylpropan-1-ol H H – C – C – C – C – OH H H H H butan-1-ol Primary, secondary, tertiary • Determined by number of alkyl groups attached to the same carbon as -OH • Distinguished by Lucas reagent (ZnCl2 in conc HCl) – no visible reaction: primary alcohol – solution turns cloudy in 3-5 minutes: secondary alcohol – solution turns cloudy immediately, and/or phases separate: tertiary or benzyl alcohol Primary alcohols • are those alcohols in which the carbon atom to which the hydroxyl group ( OH) is attached has only one carbon attached to it. (usually at the end of the chain) • I.e. the carbon with OH group is bonded to only one other carbon atom. E.g. Secondary alcohols • are those alcohols in which the carbon atom to which the hydroxyl group ( OH) is attached has two carbons attached to it • E.g. Butan-2-ol Tertiary alcohols Tertiary alcohols • are those alcohols in which the carbon atom to which the hydroxyl group ( OH) is attached has three carbons attached to it Optical Isomers A form of stereoisomerism • In stereoisomerism, the atoms making up the isomers are joined up in the same order, but still manage to have a different spatial arrangement. Why optical isomers? • Optical isomers are named like this because of their effect on plane polarised light. • Simple substances which show optical isomerism exist as two isomers known as enantiomers. Butan-2-ol