* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Asymmetric hydrogenation wikipedia , lookup
Asymmetric induction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Kinetic resolution wikipedia , lookup
Discodermolide wikipedia , lookup
Polythiophene wikipedia , lookup
Elias James Corey wikipedia , lookup
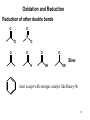
Hydrogenation wikipedia , lookup
Hydroformylation wikipedia , lookup
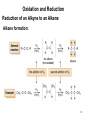
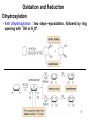
Chapter 12 Oxidation and Reduction 1 Chapter 4 Oxidation • Oxidation results in an increase in the number of C—Z bonds; or • Oxidation results in a decrease in the number of C—H bonds. • Reduction results in a decrease in the number of C—Z bonds; or • Reduction results in an increase in the number of C—H bonds. 2 Oxidation and Reduction Introduction X X • Sometimes two carbon atoms are involved in a single oxidation or reduction reaction, and the net change in the number of C—H or C—Z bonds at both atoms must be taken into account. 3 Oxidation and Reduction Reducing Agent: one that is oxidized • Addition of molecular hydrogen: with a metal catalyst. • Addition of two protons and two electrons: H2 = 2H+ + 2e-. dissolving metal reductions. • Addition of a hydride (H-) and a proton (H+): sodium borohydride (NaBH4) lithium aluminum hydride (LiAlH4). 4 Oxidation and Reduction Reduction of Alkenes—Catalytic Hydrogenation • catalytic hydrogenation : metal catalyst is required • catalyst : Pd, Pt, or Ni, adsorbed onto a finely divided inert solid, such as charcoal. heterogeneous reaction • H2 adds in a syn fashion. 5 Oxidation and Reduction Reduction of Alkenes—Catalytic Hydrogenation • When hydrogenation of two alkenes gives the same alkane, the more stable alkene has the smaller heat of hydrogenation. 6 Oxidation and Reduction Catalytic Hydrogenation : reversible ! 7 Oxidation and Reduction Reduction of Alkenes—Catalytic Hydrogenation • The mechanism explains two facts about hydrogenation: 8 Application : Structural Determination C8H12 H2, cat. C8H14 9 Oxidation and Reduction Reduction of other double bonds O O N Cl O O O O O Slow H OH OR Inert except with stronger catalyst like Raney-Ni 10 Oxidation and Reduction Reduction of Alkynes 11 Oxidation and Reduction Reduction of an Alkyne to an Alkane Alkane formation: 12 Oxidation and Reduction Reduction of an Alkyne to a Cis Alkene • To stop at a cis alkene, a less active Pd catalyst is used— Pd adsorbed onto CaCO3 with added lead(II) acetate and quinoline. This is called Lindlar’s catalyst. 13 Oxidation and Reduction Reduction of an Alkyne to a Cis Alkene • Reduction of an alkyne to a cis alkene is a stereoselective reaction, because only one stereoisomer is formed. 14 Oxidation and Reduction Reduction of an Alkyne to a Trans Alkene • dissolving metal reduction (such as Na in NH3), forms a trans alkene. adding electrons one at a time. 15 Reduction of an Alkyne to a Trans Alkene: Mechanism 16 Oxidation and Reduction Summary of Alkyne Reductions 17 Oxidation and Reduction Reduction of Polar C—X Bonds • Alkyl halides can be reduced to alkanes with LiAlH4. • Epoxide rings can be opened with LiAlH4 to form alcohols. 18 Oxidation and Reduction Reduction of Polar C—X Bonds • This reaction follows an SN2 mechanism. • Unhindered CH3X and 1° alkyl halides are more easily reduced than more substituted 2° and 3° halides. • In unsymmetrical epoxides, nucleophilic attack of H¯ (from 19 LiAlH4) occurs at the less substituted carbon atom. Summary of Reductions 20 Oxidation and Reduction Oxidizing Reactions 21 Oxidation and Reduction Oxidizing Agents (that deliver oxygen atom or take hydrogen atom) • There are two main categories of oxidizing agents: 1. Reagents that contain an oxygen-oxygen bond 2. Reagents that contain metal-oxygen bonds • • Oxidizing agents containing an O—O bond include O2, O3 (ozone), H2O2 (hydrogen peroxide), (CH3)COOH (tert-butyl hydroperoxide), and peroxyacids. Peroxyacids (or peracids) have the general formula RCO3H. 22 Oxidation and Reduction Oxidizing Agents • • Mostly chromium +6 (six Cr—O bonds) or manganese +7 (seven Mn—O bonds). Common Cr6+ reagents include CrO3 and sodium or potassium dichromate (Na2Cr2O7 and K2Cr2O7). Pyridinium chlorochromate (PCC) is a more selective Cr6+ oxidant. • • KMnO4 (potassium permanganate), MnO2. OsO4 (osmium tetroxide) and Ag2O [silver(I) oxide]. 23 Oxidation and Reduction Oxidation of Alcohols • Alcohols are oxidized to a variety of carbonyl compounds. 24 Oxidation and Reduction Oxidation of Alcohols: mostly chromium reagents • CrO3, Na2Cr2O7, and K2Cr2O7 are strong, nonselective oxidants used in aqueous acid (H2SO4 + H2O). • PCC is soluble in CH2Cl2 (dichloromethane) and can be used without strong acid present, making it a more selective, milder oxidant. 25 Oxidation and Reduction Oxidation of 2° Alcohols • Any of the Cr6+ oxidants effectively oxidize 2° alcohols to ketones. 26 Oxidation and Reduction Oxidation of 1° Alcohols • 1° Alcohols are oxidized to either aldehydes carboxylic acids, depending on the reagent. 27 or Oxidation and Reduction Oxidation of 1° Alcohols 28 Oxidation and Reduction Epoxidation • Epoxidation is the addition of a single oxygen atom to an alkene to form an epoxide. • Epoxidation is typically carried out with a peroxyacid. 29 Oxidation and Reduction Epoxidation • more substituted, electron rich alkenes react faster. • a cis alkene gives an epoxide with cis substituents. A trans alkene gives an epoxide with trans substituents. • This reaction is stereospecific because cis and trans alkenes yield different stereoisomers as products. 30 Epoxidation Attack from below Attack from above CH3 C C CH3 O mCPBA CH3 H H H cis-2-Butene C * C* + CH3 H CH3 H CH3 * H C C* O Achiral meso compound CH3 H C C H CH3 mCPBA Attack from below Attack from above O CH3 H C* C* H CH3 + CH3 H trans-2-Butene Enantiomers * C * C O H CH3 Oxidation and Reduction The Synthesis of Disparlure • • • • Disparlure, the sex pheromone of the gypsy moth. Carterpillars of gypsy moth eats leaves of broadleaf trees Use: attract and trap male moths. Retrosynthetic analysis of disparlure illustrates three key operations: 32 Oxidation and Reduction The Synthesis of Disparlure 33 Oxidation and Reduction Dihydroxylation • Dihydroxylation is the addition of two hydroxy groups to a double bond, forming a 1,2-diol or glycol. 34 Oxidation and Reduction Dihydroxylation • Anti dihydroxylation : two steps—epoxidation, followed by ring opening with ¯OH or H3O+. 35 Oxidation and Reduction Dihydroxylation • Syn hydroxylation : with either KMnO4 or OsO4. Insoluble in organic solvent 36 Oxidation and Reduction Dihydroxylation • Each reagent adds two oxygen atoms in a syn fashion. 37 Oxidation and Reduction Dihydroxylation : catalytic version • Dihydroxylation with a catalytic amount of OsO4 : the oxidant Nmethylmorpholine N-oxide (NMO) 38 Oxidation and Reduction Oxidative Cleavage of Alkenes • Oxidative cleavage forms two carbonyl compounds. Cleavage with ozone (O3) is called ozonolysis. 39 Oxidative Cleavage of Alkenes : mechanism 40 Oxidation and Reduction Oxidative Cleavage of Alkenes • Ozonolysis of dienes or other polyenes results in oxidative cleavage of all C=C bonds. • It is important to note that when oxidative cleavage involves a double bond that is part of a ring, the ring opens up affording a single chain with two carbonyls at the carbons where the double bonds were originally. 41 Oxidation and Reduction Oxidative Cleavage of Alkynes • Alkynes undergo oxidative cleavage of the and both bonds. • Internal alkynes are oxidized to carboxylic acids (RCOOH). 42 Oxidation and Reduction Green Chemistry • Green chemistry : use of environmentally benign methods to synthesize compounds i.e. to use safer reagents and less solvent, and develop reactions that form fewer by-products and generate less waste. • many oxidation methods use toxic reagents (such as OsO4 and O3) and corrosive acids such as H2SO4, or generate carcinogenic by-products (such as Cr3+). One methods uses a polymer supported Cr3+ reagent— Amberlyte A-26 resin-HCrO4—that avoids the use of strong acid, and forms a Cr3+ by-product that can easily be removed from the product by filtration. 43 Oxidation and Reduction Green Chemistry • With Amberlyst A-26 resin-HCrO4¯, 1° alcohols are oxidized to aldehydes and 2° alcohols are oxidized to ketones. 44 The Oxidation of Ethanol • In the body, ingested ethanol is oxidized in the liver first to CH3CHO (acetaldehyde), and then to CH3COO¯ (the acetate anion). • This oxidation is catalyzed by alcohol dehydrogenase. • If more ethanol is ingested than can be metabolized, the concentration of acetaldehyde increases. Acetaldehyde, which is toxic, is responsible for the feelings associated with a hangover. • If methanol is ingested, it is metabolized by the same enzyme to formaldehyde and formic acid. These compounds are extremely toxic since they cannot be used by the body. Blood pH decreases, and blindness and death can follow. 45 Homework 12.40, 12.42, 12.43, 12.50, 12.51, 12.52, 12.58, 12.60, 12.61 46 Preview of Chapter 13 and 14 Organic Structural Identification : NMR, IR, UV, MS What information can you obtain from MS? What does IR spectroscopy measure? What does 1H-NMR provide to determine the molecular structure? 47