* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture 2
Citric acid cycle wikipedia , lookup
Oxidation state wikipedia , lookup
Transition state theory wikipedia , lookup
Hydrogen-bond catalysis wikipedia , lookup
Chemical reaction wikipedia , lookup
Electrolysis of water wikipedia , lookup
Flux (metallurgy) wikipedia , lookup
Atomic theory wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Computational chemistry wikipedia , lookup
IUPAC nomenclature of inorganic chemistry 2005 wikipedia , lookup
Photoredox catalysis wikipedia , lookup
Cation–pi interaction wikipedia , lookup
History of molecular theory wikipedia , lookup
Geochemistry wikipedia , lookup
Acid dissociation constant wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Nucleophilic acyl substitution wikipedia , lookup
Atomic orbital wikipedia , lookup
Electrochemistry wikipedia , lookup
Acid strength wikipedia , lookup
Inorganic chemistry wikipedia , lookup
Hypervalent molecule wikipedia , lookup
Surface properties of transition metal oxides wikipedia , lookup
Chemical bond wikipedia , lookup
Stability constants of complexes wikipedia , lookup
Jahn–Teller effect wikipedia , lookup
Molecular orbital wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Bent's rule wikipedia , lookup
Electron configuration wikipedia , lookup
Metallic bonding wikipedia , lookup
Biochemistry wikipedia , lookup
Coordination complex wikipedia , lookup
Molecular orbital diagram wikipedia , lookup
Metalloprotein wikipedia , lookup
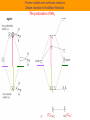
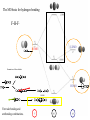
y N H H H x y 3 s orbitals of 3 H atoms around C3 can form 3 "group orbitals" (SALC AO's) e.g. a + b + c is of A1 symmetry in C3v y x s and pz orbitals of N atom above the center of the triangle are also A1 in C3v x = c1N2s + c2Npz + c3(a + b +c) (1a1) + 2 others (2a1 and 3a1) This is now one (group) orbital! y N H y H 3 s orbitals of 3 H atoms around C3 can form a pair of "group orbitals" a - b) and a - b - c) of E symmetry in C3v H x y x y x x px, py orbitals of N atom above the center of the triangle are also E in C3v = c1Npx + c2Npy + c3(a - b) + c4(2a - b - c ) (1e) + another pair 2e : N H H H Homo is lone pair Fundamentals of Molecular Orbital Theory Main concepts Main Skills MO = LCAO Mathematical for of the AO Many electron problem One electron proble Eigenequation Perturbation Theory Point Groups Symmetry control of orbital formation Simple Huckel Theory SALC PhotoElectron Spectroscopy Orbital mixing IR and Raman Particle in a box Using Simple Huckel Theory Small Molecules Interpreting PES Assigning a point group to a molecule Obtaining reducible representations Reducing to irreducible representations. Projection Operator to obtain SALC Interaction Diagrams Obtaining allowed vibrational excitations. Acid-base and donor-acceptor chemistry Hard and soft acids and bases Classical concepts Arrhenius: • acids form hydrogen ions H+ (hydronium, oxonium H3O+) in aqueous solution • bases form hydroxide ions OH- in aqueous solution • acid + base salt + water e.g. HNO3 + KOH KNO3 + H2O Brønsted-Lowry: • acids tend to lose H+ • bases tend to gain H+ • acid 1 + base 2 base 1 + acid 2 (conjugate pairs) H3O+ + NO2- H2O + HNO2 NH4+ + NH2- NH3 + NH3 In any solvent, the reaction always favors the formation of the weaker acids or bases The Lewis concept is more general and can be interpreted in terms of MO’s CO Remember that frontier orbitals define the chemistry of a molecule CO is a a p-acceptor and s-donor d+ d- C O M C O C O M Acids and bases (the Lewis concept) A base is an electron-pair donor An acid is an electron-pair acceptor acid adduct base Lewis acid-base adducts involving metal ions are called coordination compounds (or complexes) NH3 Frontier orbitals and acid-base reactions N-H s* Remember the NH3 molecule N-H s Frontier orbitals and acid-base reactions Simple example of Acid/Base Reaction. Now more detail… Frontier orbitals and acid-base reactions Simple example of Acid/Base Reaction. The protonation of NH3 again (Td) (C3v) But remember that there must be useful overlap (same symmetry) and similar energies to form new bonding and antibonding orbitals What reactions take place if energies are very different? Frontier orbitals and acid-base reactions Very different energies like A-B or A-E get reaction but no adducts form Similar energies like A-C or A-D adducts form A base has an electron-pair in a HOMO of suitable symmetry to interact with the LUMO of the acid The MO basis for hydrogen bonding F-H-F- As before…. MO diagram derived from atomic orbitals (using F…….F group orbitals + H orbitals) Bonding e Non-bonding e But it is also possible from HF + F-, Hydrogen Bonding First form HF HOMO-LUMO of HF for s interaction Non-bonding (no symmetry match) Non-bonding (no E match) The MO basis for hydrogen bonding F-H-F- LUMO HOMO Formation of the orbitals HOMO HOMO First take bonding and antibonding combinations. Similarly for unsymmetrical B-HA Total energy of B-H-A lower than the sum of the energies of reactants Poor energy match, little or no Hbonding e.g. CH4 + H2O Good energy match, strong H-bonding e.g. CH3COOH + H2O Very poor energy match no adduct formed H+ transfer reaction e.g. HCl + H2O Ralph Pearson introduced the Hard Soft [Lewis] Acid Base (HSAB) principle in the early nineteen sixties, and in doing so attempted to unify inorganic and organic reaction chemistry. The impact of the new idea was immediate, however over time the HSAB principle has rather fallen by the wayside while other approaches developed at the same time, such as frontier molecular orbital (FMO) theory and molecular mechanics, have flourished. The Irving-Williams stability series (1953) pointed out that for a given ligand the stability of dipositive metal ion complexes increases: It was also known that certain ligands formed their most stable complexes with metal ions like Al3+, Ti4+ and Co3+ while others formed stable complexes with Ag+, Hg2+ and Pt2+. In 1958 Ahrland classified metal cations as Type A and Type B, where: Type A metal cations included: • Alkali metal cations: Li+ to Cs+ • Alkaline earth metal cations: Be2+ to Ba2+ • Lighter transition metal cations in higher oxidation states: Ti4+, Cr3+, Fe3+, Co3+ • The proton, H+ Type B metal cations include: • Heavier transition metal cations in lower oxidation states: Cu+, Ag+, Cd2+, Hg+, Ni2+, Pd2+, Pt2+. Ligands were classified as Type A or Type B depending upon whether they formed more stable complexes with Type A or Type B metals: Type A metals prefer to bind to Type A ligands and Type B metals prefer to bind to Type B ligands These empirical (experimentally derived) rules tell us that Type A metals are more likely to form oxides, carbonates, nitrides and fluorides, Type B metals are more likely to form phosphides, sulfides and selinides. This type of analysis is of great economic importance because some metals are found in nature as sulfide ores: PbS, CdS, NiS, etc., while other are found as carbonates: MgCO3 and CaCO3 and others as oxides: Fe2O3 and TiO2. In the nineteen sixties, Ralph Pearson developed the Type A and and Type B logic by explaining the differential complexation behaviour of cations and ligands in terms of electron pair donating Lewis bases and electron pair accepting Lewis acids: Lewis acid + Lewis base Lewis acid/base complex Pearson classified Lewis acids and Lewis bases as hard, borderline or soft. According to Pearson's hard soft [Lewis] acid base (HSAB) principle: Hard [Lewis] acids prefer to bind to hard [Lewis] bases and Soft [Lewis] acids prefer to bind to soft [Lewis] bases At first sight, HSAB analysis seems rather similar to the Type A and Type B system. However, Pearson classified a very wide range of atoms, ions, molecules and molecular ions as hard, borderline or soft Lewis acids or Lewis bases, moving the analysis from traditional metal/ligand inorganic chemistry into the realm of organic chemistry. Hard Acids Hard Bases Borderline Acids Borderline Bases Soft Acids Soft Bases Most metals are classified as Hard acids or acceptors. Exceptions: acceptors metals in red box are always soft . Green boxes are soft in low oxidation states. Solubilities: (S-H)AgF > AgCl > AgBr >AgI (S-S) But…… LiBr > LiCl > LiI > LiF Orange boxes are soft in high oxidation states. Log K for complex formation hard soft softness Most metals are classified as Hard acids or acceptors. Exceptions: acceptors metals in red box are always soft . Green boxes are soft in low oxidation states. Solubilities: (S-H)AgF > AgCl > AgBr >AgI (S-S) But…… LiBr > LiCl > LiI > LiF Orange boxes are soft in high oxidation states. Chatt’s explanation: soft metals ACIDS have d electrons available for p-bonding Model: Base donates electron density to metal acceptor. Back donation, from acid to base, may occur from the metal d electrons into vacant orbitals on the base. Higher oxidation states of elements to the right of transition metals have more soft character. There are electrons outside the d shell which interfere with pi bonding. In higher oxidation states they are removed. For transition metals: high oxidation states and position to the left of periodic table are hard low oxidation states and position to the right of periodic table are soft Soft BASE molecules or ions that are readily polarizable and have vacant d or π* orbitals available for π back-bonding react best with soft metals Tendency to complex with hard metal ions N >> P > As > Sb O >> S > Se > Te F > Cl > Br > I Tendency to complex with soft metal ions N << P > As > Sb O << S > Se ~ Te F < Cl < Br < I The hard-soft distinction is linked to polarizability, the degree to which a molecule or ion may be easily distorted by interaction with other molecules or ions. Hard acids or bases are small and non-polarizable Hard acids are cations with high positive charge (3+ or greater), or cations with d electrons not available for π-bonding Soft acids are cations with a moderate positive charge (2+ or lower), Or cations with d electrons readily availbale for π-bonding The larger and more massive an ion, the softer (large number of internal electrons shield the outer ones making the atom or ion more polarizable) Soft acids and bases are larger and more polarizable For bases, a large number of electrons or a larger size are related to soft character Hard acids tend to react better with hard bases and soft acids with soft bases, in order to produce hard-hard or soft-soft combinations In general, hard-hard combinations are energetically more favorable than soft-soft An acid or a base may be hard or soft and at the same time it may be strong or weak Both characteristics must always be taken into account e.g. If two bases equally soft compete for the same acid, the one with greater basicity will be preferred but if they are not equally soft, the preference may be inverted Fajans’ rules 1. For a given cation, covalent character increases with increasing anion size. F<Cl<Br<I 2. For a given anion, covalent character increases with decreasing cation size. K<Na<Li 3. The covalent character increases with increasing charge on either ion. 4. Covalent character is greater for cations with non-noble gas electronic configurations. A greater covalent character resulting from a soft-soft interaction is related to lower solubility, color and short interionic distances, whereas hard-hard interactions result in colorless and highly soluble compounds Examples •Harder nucleophiles like alkoxide ion, R-O–, attack the acyl (carbonyl) carbon. •Softer nucleophiles like the cyanide ion, NC–, and the thioanion, R-S–, attack the "beta" alkyl carbon Further Development Pearson and Parr defined the chemical hardness, h, as the second derivative for how the energy with respect to the number of electrons. Expanding with a three point approximation Related to Mulliken electronegativity softness s 1 h IA 2 Energy levels for halogens and relations between , h and HOMOLUMO energies Chemical Hardness, , in electron volt Acids Bases + Hydrogen H Aluminum Al Lithium Li Scandium Sc Sodium Na Lanthanum La Zinc Zn - infinite Fluoride F 45.8 Ammonia NH3 35.1 hydride H 24.6 carbon monoxide CO 21.1 hydroxyl OH 15.4 cyanide CN 5.3 10.8 phosphane PH3 5.0 Carbon dioxide CO2 10.8 nitrite NO2 Sulfur dioxide SO2 5.6 Hydrosulfide SH Iodine I2 Methane CH3 3+ + 3+ + 3+ 2+ 3.4 7 6.8 - 6.8 6.0 - 5.6 - - - 4.5 4.1 - 4.0