* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Organometallic Catalysts
George S. Hammond wikipedia , lookup
Discodermolide wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
Asymmetric induction wikipedia , lookup
Kinetic resolution wikipedia , lookup
Petasis reaction wikipedia , lookup
Stille reaction wikipedia , lookup
Polythiophene wikipedia , lookup
Cracking (chemistry) wikipedia , lookup
Ene reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Fischer–Tropsch process wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Asymmetric hydrogenation wikipedia , lookup
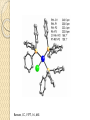
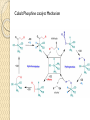
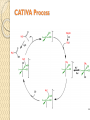
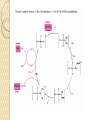
Organometallic Catalysts Presenter : Saber Askari Advisor : Dr.Mirzaaghayan May 2012 Contents : The basis for catalysis Catalytic Cycle History Mechanistic Concept Homogeneous Catalysis Wilkinson’s Catalyst Asymmetric hydrogenation Hydroformylation Monsanto Acetic acid Process CATIVA Process Wacker Process Heterogeneous Catalysis Ziegler-Natta Catalyst The basis for catalysis A Catalyst is a substance which speed up the rate of a reaction without itself being consumed. A catalyst lowers the activation energy for a chemical reaction The catalyzed reaction goes by a multistep mechanism in which the metal stabilizes intermediates that are stable only when bound to metal . Importance of catalysis Many major industrial chemicals are prepared with the aid of catalysts Many fine chemicals are also made with the aid of catalysts – Reduce cost of production – Lead to better selectivity and less waste Catalytic Cycle The catalytically active species must have a vacant coordination site to allow the substrate to coordinate The establishment of a reaction mechanism is always a difficult task. It is even harder to definitively establish a catalytic cycle as all the reactions are going on in parallel! Late transition metals are privileged catalysts (from 16e species easily) In general , the total electron count alternates between 16 and 18 One of the catalytic steps in the cycle is rate-determining History Fundamental Reaction Mechanistic Concept Homogeneous Catalysis Wilkinson`s Catalyst : Olefin Hydrogenation Hydroformylation Monsanto Acetic acid Process Wacker Process Heterogeneous Catalysis Ziegler-Natta Catalysts Homogeneous Catalysis Homogenous catalysts are used when selectivity is critical and product-catalyst separation problems can be solved. Advantages : Relatively high specificity Relatively low reaction temperatures Generally far more selective for a single product far more easily studied from chemical & mechanistic aspects far more active Disadvantages : o far more difficult for achieving product/catalyst separations Catalytic steps in homogeneous reactions Most catalytic process can be built up from a small number of different types of step – Association / dissociation of a ligand » requires labile complexes – Insertion and elimination reactions – Nucleophilic attack on a coordinated ligand – Oxidation and reduction of a metal center – Oxidative addition / reductive elimination Wilkinson’s Catalyst: RhCl(PPh3)3 was the first highly active homogeneous hydrogenation catalyst and was discovered by Geoffrey Wilkinson (Nobel prize winner for Ferrocene) in 1964. Wilkinson’s Catalyst is a Rh(I) complex, Rh(PPh3)3Cl containing three phosphine ligands and one chlorine. As a result of the olefin insertion (hydrogen migration) we obtain a Rh (III), 16e-, five coordinate species. A solvent occupies the sixth coordination site to take it to a 18e- species. Reductive elimination occurs to give the hydrogenated product and the catalytically active species. Bennett , IC , 1977 , 16 , 665 Olefin Hydrogenation using Wilkinson’s Catalyst The complex RhCl(PPh3)3 (also known as Wilkinson’s catalyst) became the first highly active homogeneous hydrogenation catalyst that compared in rates with heterogeneous counterparts. Wilkinson, J. Chem. Soc. (A) 1966, 1711 Hydrogenation mechanism Steps: (1) H2 addition, (2) alkene addition, (3) migratory insertion, (4) reductive elimination of the alkane, regeneration of the catalyst Halpern, Chem. Com. 1973, 629; J. Mol. Cat. 1976, 2, 65; Inorg. Chim. Acta. 1981, 50, 11 Wilkinson’s catalyst selectivity The rate of hydrogenation depends on : (a) presence of a functional group in the vicinity of the C=C bond (b) degree of substitution of the C=C fragment Wilkinson’s catalyst selectivity Hydrogenation is stereoselective: Rh preferentially binds to the least sterically hindered face of the olefin: Wilkinson’s catalyst selectivity Cis-disubstituted C=C react faster than trans-disubstituted C=C: Schneider, JOC 1973, 38, 951 Cationic catalysts Cationic catalysts are the most active homogeneous hydrogenation catalysts developed so far: Halpern’s mechanism of hydrogenation for cationic Rh catalysts with bidentate phosphines Halpern, Science 1982, 217, 401. Steps: (1) alkene addition, (2) (2) H2 addition, (3) migratory insertion, (4) reductive elimination of the alkane, regeneration of the catalyst. Asymmetric hydrogenation A variety of bidentate chiral diphosphines have been synthesized and used to make amino acids by hydrogenation of enamides: Burk, Acc. Chem. Res 2000, 33, 363. • Synthesis of derivative of L-dihydroxyphenylalanine Chiral hydrogenation catalysts Catalysts similar to Wilkinson’s but using chiral phosphine ligands have been used for the asymmetric hydrogenation of small molecules . – Important in the fine chemicals /pharmaceutical industry Noles and Nyori received the 2001 chemistry Nobel prize for the development of asymmetric hydrogenation catalysis Intermediates in Noyori’s transfer hydrogenation Knowles, JACS 1975, 97, 2567. Lanthanide Hydrogenation Catalysts Tobin Marks reported the extraordinary activity of (Cp*2LuH)2 for the hydrogenation of alkenes and alkynes. The monometallic complex catalyzes the hydrogenation of 1hexene with a TOF = 120,000 hr-1 at 1 atm H2, 25ºC!! This is one of the most active hydrogenation catalysts known. Catalytically active species With bidentate ligands, olefin coordination can precede oxidative addition of H2 (S = methanol, ethanol, acetone). Halpern, JACS 1977, 99, 8055 Hydroformylation The reaction of an alkene with carbon monoxide and hydrogen, catalyzed by cobalt or rhodium salts to form an aldehyde is called hydroformylation. Hydroformylation was discovered by Otto Roelen in 1938. Heck , JACS,1961,83,4023 Cobalt Phosphine modified catalyst Cobalt Phosphine catalyst Mechanism Monsanto Acetic acid Process 1960 basf 1966 monsanto CATIVA Process CATIVA Process Wacker Process This is one of the earliest industrial processes developed in Germany for the conversion of ethylene into acetaldehyde. Wacker process is more complex than the other catalytic processes described above. Heterogeneous Catalysis Heterogeneous catalysts dominate chemical and petrochemical industry: ~ 95% of all chemical processes use heterogenous catalysts. Ziegler-Natta Catalysis for the Polymerization of olefins Polymers are large molecules with molecular weights in the range of 104 to 106. These consist of small building units known as monomers For example polyethylene is made up of ethylene monomers In all of these cases a single monomer is repeated several times in the polymer chain. The number of repeating units determines the molecular weight of the polymer. The German chemist Karl Ziegler (1898-1973) discovered in 1953 that when TiCl3(s) and AlEt3 are combined together they produced an extremely active heterogeneous catalyst for the polymerization of ethylene at atmospheric pressure. Giulio Natta (1903-1979), an Italian chemist, extended the method to other olefins like propylene and developed variations of the Ziegler catalyst based on his findings on the mechanism of the polymerization reaction. The Ziegler-Natta catalyst family includes halides of titanium, chromium, vanadium, and zirconium, typically activated by alkyl aluminum compounds Ziegler and Natta received the Nobel Prize in Chemistry for their work in 1963. There are typically three parts to most polymerizations: Thanks for your attention