* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download R e v i s i ó n
Survey
Document related concepts
Molecular mimicry wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Immune system wikipedia , lookup
Major urinary proteins wikipedia , lookup
Adaptive immune system wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Lymphopoiesis wikipedia , lookup
Innate immune system wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
X-linked severe combined immunodeficiency wikipedia , lookup
Transcript
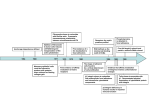
Revisión VOL. 20 / N ÚM. 2 / A BRIL-JUNIO 2001 2001; PP 78-87 INMUNOLOGÍA, Integrins: what are they doing on leukocytes in vivo? A. G. ARROYO Servicio de Inmunología. Hospital de la Princesa. Madrid INTEGRINAS: ¿QUÉ ESTÁN HACIENDO EN LOS LEUCOCITOS IN VIVO? RESUMEN Las integrinas constituyen una familia importante de receptores de adhesión que pueden reconocer tanto componentes de la matriz extracelular como ligandos celulares. Distintas aproximaciones experimentales in vitro han mostrado que las integrinas pueden jugar un papel importante en diversas funciones leucocitarias. Recientemente, la generación de ratones manipulados genéticamente ha proporcionado una herramienta muy útil para diseccionar las funciones que estos receptores de adhesión desempeñan in vivo en los leucocitos. De forma interesante, el análisis de ratones deficientes en alguna integrina ha revelado funciones insospechadas para estos receptores como es el caso del papel de las integrinas β1 en la migración de progenitores hematopoyéticos o del papel de la integrina αMβ2 en la apoptosis de neutrófilos. Sin embargo, otros hallazgos en estos ratones han confirmado las funciones que se habían sugerido previamente por otros abordajes como es el papel de algunas integrinas en el tráfico leucocitario o en la migración leucocitaria durante la inflamación. Finalmente, los ratones deficientes en integrinas pueden servir en algunos casos como modelos animales de enfermedades humanas tales como la deficiencia de adhesión leucocitaria o el asma. ABSTRACT Integrins constitute a major family of adhesion receptors that recognise extracellular matrix components as well as cellular ligands. They have been shown to play roles in a variety of leukocyte functions by different in vitro approaches. Recently, the generation of genetically manipulated mice has provided a useful tool to dissect the in vivo functions that these adhesion receptors play on leukocytes. Interestingly, some of the revealed functions from the analysis of deficient mice have been unsuspected such as the role of β1 integrins in homing of hematopoietic progenitors or the role of αMβ2 in neutrophil apoptosis. However, other findings in these mice have confirmed the functions that had previously been suggested by other approaches including the role of distinct integrins in lymphocyte homing or in leukocyte migration during inflammation. Finally, integrin deficient mice can provide in some cases animal models for human diseases such as the leukocyte adhesion deficiency or the asthma. PALABRAS CLAVE: Integrina/ Adhesión/ Leucocito/ rratón/ Knockout. KEY WORDS: Integrins/ Adhesion/ Leukocyte/ Mouse/ Knockout. INTRODUCTION poietic stroma had been shown to be important for leukocyte development (1). Once lymphocytes mature, they recirculate constantly through secondary lymphoid organs to exert their surveillance function. Moreover, both lymphocytes and myeloid cells need to attach to the endothelium and transmigrate during inflammation to reach L 78 eukocyte development occurs in the foetal liver during the embryonic life and in the bone marrow during postnatal life except for T lymphocytes that develop in the thymus. Interactions with the hemato- INMUNOLOGÍA the inflammatory foci (2). Leukocytes that normally circulate as non-adherent cells are able to attach to the endothelium and stroma during all these processes. To this purpose, they use adhesion receptors including integrins. The integrin family is composed of at least 24 heterodimers formed by the association of 8β subunits and 18α subunits (3). Integrins constitute an important family of adhesion receptors that recognise extracellular matrix (ECM) proteins as well as cellular ligands (4). Classically, subfamilies have been distinguished depending on the β subunit present in the heterodimer. Thus, β1 subfamily contains members that mainly recognize ECM proteins such as fibronectin, collagen, laminin, and others except α4β1 and α9β1 that can also bind the cellular ligand VCAM-1. The β7 subfamily comprises the heterodimers α4β 7 and αEβ7 that bind MAdCAM-1 and E-cadherin, respectively. The β2 subfamily constitutes the leukocyte integrins since they are expressed on this cellular subtype. Other β chains (β3, β5, β6, and β8) associate to αv and β4 to α6. Integrins are important as adhesion receptors and also as linkers between the cell membrane and the cytoskeleton and as signal transducers (5). As mentioned, β2 integrins, composed by four heterodimers, are mostly expressed on different leukocyte subtypes (T and B lymphocytes, and myeloid cells) and they recognise different ligands such as ICAM-1, -2, and –3, iC3b, and others (6). The β1 subfamily is finely regulated in expression and function on the hematopoietic lineage. Thus, α4β1 and α5β1 are expressed early during hematopoiesis and later in lymphocytes and polymorphonuclear subsets. Other members are activated upon T lymphoid activation (7). Different in vitro approaches using monoclonal antibodies (mAbs), ligands and other tools had suggested important roles for integrins during leukocyte development, traffic and activation. Genetic manipulation of mice has provided an useful tool to elucidate the in vivo roles that integrins might be playing in leukocyte physiology (8). In this review, we will try to summarise the major findings obtained from the analysis of mice deficient and chimeric for integrins related to defects in any aspect of leukocyte physiology including development, homing and migration, activation and others. We will also comment specific knockout mice that might be used as models for human immune disease. INTEGRINS DURING IN VIVO LEUKOCYTE DEVELOPMENT Leukocyte progenitors need to migrate to different organs and to interact with the hematopoietic stroma to successfully complete their develop- A. G. ARROYO ment. Adhesion receptors including integrins have been suggested to play roles during this process (for review see 9). Thus, anti-β1 antibodies decreased the formation of colony-forming units and the lodging of progenitors in the spleen and anti-α4 antibodies could inhibit B lymphocyte development and delayed myeloid development in vitro (10-12). Knockout and chimeric mice have revealed interesting in vivo functions for integrins during hematopoiesis including leukocyte development. Homing failure of progenitors to hematopoietic organs in the absence of 1 integrins Homing of hematopoietic stem cells (HSCs) to hematopoietic organs is required for the establishment of hematopoiesis during embryogenesis and after bone marrow transplantation. Mice chimeric or deficient for the β1 subunit in an inducible manner have shown that β1 integrins are critical on foetal as well as adult HSCs for homing to appropriate environments including foetal liver, thymus, spleen, or bone marrow. However, the potential of β1 null cells to differentiate in vitro (in foetal organ cultures or in adult spleen context) is not affected. Thus, β1 integrins are crucial adhesion molecules for the homing of HSCs (13,14) resulting in a complete blockade of hematopoiesis in vivo. With regard to the β1 partner responsible for these defects, no single integrin subunit knockout mouse displays the same phenotype than β1 integrin deficient mice. It might be either a combination of a subunits that individually can be deleted without effect, or an as yet unknown subunit, maybe developmentally regulated, that heterodimerizes with β1 integrin and mediates the functions essential for the homing of HSCs to the hematopoietic organs. 4 integrins are essential to regulate the proliferation/differentiation balance of hematopoietic progenitors in vivo α4 integrins had been suggested to play roles in migration, attachment, transmigration, proliferation and differentiation of hematopoietic progenitors in vitro (9), but their role in vivo remained undefined. The first characterisation of α4 integrin chimeric mice showed that α4 integrins were essential for B lymphocyte development in the bone marrow during the postnatal life (15). T lymphocyte development appeared to be normal during embryonic development and was only impaired thereafter likely due to a defect at the progenitor level within the bone marrow environment. Other lineages such as monocytes and natural killer cells seemed 79 INTEGRINS: WHAT ARE THEY DOING ON LEUKOCYTES IN VIVO? VOL. to be less affected by the absence of α4 integrins. A more detailed analysis of the hematopoiesis during all stages of mouse development in α4 chimeric mice, showed that α4 integrins are essential for development of all lineages in the foetal liver and the dependence is greater at later stages in the bone marrow (16). Homing of progenitors to foetal liver or bone marrow, however, seems to be normal. Interestingly, transmigration of the progenitors through the bone marrow or foetal liver stroma is decreased in the absence of α4 integrins resulting in a defect in proliferation of the hematopoietic progenitors within these environments. These defects allow the differentiation of mature blood cells but at much lower numbers in the absence of these receptors. Thus, α4 integrins are demonstrated to be essential for regulation of the proliferation/differentiation balance of hematopoietic progenitors within the foetal liver and bone marrow microenvironments. In figure 1, a working model for the roles of α4 integrins in regulating hematopoiesis is proposed (16). Hematopoietic progenitors normally seed and attach to the stroma. It is possible to distinguish three compartments for self-renewal, expansion, and maturation of progenitors within the stroma (17). In these stromal compartments, the- 20 NÚM . 2 / 2001 re might be a differential secretion of soluble factors such as cytokines and chemokines as well as distinct stroma-progenitor cell interactions. The balance among all these signals (18) and the competition for the niches among different progenitors would determine the fate of the hematopoietic progenitors. In the expansion stroma, progenitors would transmigrate beneath in response to chemokines, and cytokines might also facilitate this process by activating adhesion receptors such as integrins (9). In that niche, progenitors proliferate in contact with extracellular matrix proteins such as fibronectin and growth factors presented by the proteoglycans. After this expansion stage, progenitors continue differentiation into mature cells under the influence of other cytokines in the maturation compartment. In this model, α4 integrins would act as a retention signal for progenitors to remain in the expansion compartment. In the absence of α4 integrins two major steps would be impaired: first, the response to cytokines and chemokines and subsequent attachment and transmigration beneath the stroma, and second, proliferation mediated by contact with fibronectin and growth factors. As a consequence, α4-deficient progenitors would detach prematurely, skipping the expansion phase, wild type Progenitors α4β1 Stroma Chemokines α4β1 Growth Factors ECM FN FN α4 null Mature cells Progenitors α4β1 Fetal liver Stroma Spleen Bone marrow Chemokines α4β1 Growth Factors FN FN ECM Figure 1. α4 integrins are essential to control the normal balance between proliferation and differentiation of hema- topoietic progenitors in vivo. A model for regulation of the proliferation of hematopoietic progenitors by α4 integrins in vivo is proposed. α4 integrins would participate in the adhesion and subsequent transmigration of progenitors through the stroma stimulated by chemokines. Moreover, α4 integrins would also play a role in recognising growth factors presented by the extracellular matrix beneath the stroma. In the absence of α4 integrins, these two steps would be impaired resulting in skipping of the expansion phase, leading the progenitors directly to differentiation and resulting in low numbers of mature blood cells. 80 INMUNOLOGÍA and shift towards differentiation. This might result in low numbers of progenitors involved in hematopoiesis and lower yields of mature cells. α4 integrin chain can associate with β1 or β7 subunits. Since β7 deficient mice do not display any defect in leukocyte development (19), it seems that α4β1 is the responsible for these critical interactions. α4β1 can bind fibronectin and VCAM-1. It is not possible to know at this point which of the ligands is essential since VCAM-1 null mice do not have major defects in leukocyte development and fibronectin null mice are lethal very early (20). Moreover, conditional VCAM-1 deficient mice have recently been generated and they mainly show defects in migration of lymphocytes to the bone marrow and in T-cell dependent humoral immune response (21,22). These defects do not reproduce those found in α4 chimeric mice. It is possible that both ligands or as yet unknown one are involved since there is no compensation by other fibronectin receptors such as α5β1 or αvβ1 nor by other VCAM1 receptors such as α9β1, in spite of its similarities with α4β1 in structure and ligands (23), or αdβ2 that is expressed on a variety of leukocytes (24). M 2 integrin is required for mast cell development αMβ2 is a member of the β2 subfamily expressed in several leukocyte subsets. The analysis of αMβ2 null mice has surprisingly shown that it is required for the expression of normal levels of mast cells in the peritoneal cavity, peritoneal wall, and certain areas of skin (25). αMβ2 is also shown to be important for maintaining adequate cell-dependent host defence against bacterial infection since these mice exhibit increased mortality in a model of acute septic peritonitis in which it is known that host resistance rely on mast cells and complement (25). The mechanism for this regulation of resident mast cells numbers by αMβ2 is unknown. Thus, αMβ2 might be affecting migration of mast cell progenitors or proliferation and survival of differentiated mast cells rather than development itself. In summary, so far only a few integrin receptors (β1 and α4 integrins, and αMβ2) have been shown to play roles in leukocyte development in vivo. β1 subunit as the partner for multiple α chains constituting the major adhesion receptor subfamily is essential for the homing of progenitors to hematopoietic sites and its absence results in the earliest blockade in leukocyte development. α4 integrins participate at later stages when progenitors are already within the hematopoietic organs and regulate their transmigration and proliferation with a decreased numbers of mature blood cells in their absence. Finally, a more specific defect would be caused by the deficiency of αMβ2 that seems to only affect the number of mast cells. A. G. ARROYO INTEGRINS DURING IN VIVO LEUKOCYTE HOMING AND MIGRATION The most important functions of leukocytes are the surveillance of exogenous pathogens and the fight against them. In order to do so, lymphocytes need to recirculate constantly through different secondary lymphoid organs and leukocytes in general need to adhere and go through anatomic barriers including the endothelium and ECM to reach the inflammatory foci (2). Thus, leukocytes, that circulate normally as non-adherent cells in the bloodstream, need to quickly switch to an adherent state to be able to reach different sites by using adhesion receptors. Different approaches have suggested important roles for integrin receptors in leukocyte homing by binding to addressins and migration to inflammatory foci (26), but now the analysis of genetically manipulated mice have demonstrated the role that these receptors play in vivo. Integrins and homing 4, 7, and αE integrins are essential for constitution of the gut-associated lymphoid tissue The gut-associated lymphoid tissue in the intestinal mucosa represents an integrated system of secondary lymphoid organs (Peyer’s patches and lymph mesenteric nodes) and mucosal effector sites (lamina propria and the intraepithelial lymphocyte compartment located above the villus basement membrane). In vivo data from the analysis of chimeric mice have shown that α4 integrins are essential for migration of lymphocytes to Peyer’s patches (15,27). The α4 integrin chain can associate with β1 or β7 subunits. The analysis of β7 deficient mice has shown a critical role for β7 receptors in migration of lymphocytes to Peyer’s patches and an important role in trafficking to the intraepithelial intestinal compartment and lamina propria (19). The defect observed in migration to Peyer’s patches in the absence of α4β7 fits with the presence of its specific ligand, the addressin MAdCAM-1 at this site. However, α4 integrins are not critical for migration to intestinal epithelium and lamina propria, suggesting that other β7 partners might be responsible for the defects. In this regard, αE-null mice show decreased numbers of intraepithelial and lamina propria lymphocytes (28). In conclusion, α4β7 integrin is essential for migration of lymphocytes to Peyer’s patches and αEβ7 is critical for localisation of lymphocytes within the intestinal epithelium and lamina propria. It is possible that β2 integrins might also participate in the establishment of the intestinal lymphocyte compartment in vivo as it has been shown in vitro (29) but this point has not been investigated yet. 81 INTEGRINS: WHAT ARE THEY DOING ON LEUKOCYTES IN VIVO? L 2 (LFA-1) integrin participates in homing of lymphocytes to peripheral lymph nodes LFA-1 is expressed on lymphocytes, monocytes, granulocytes, and bone marrow cells. Its natural ligands include ICAM-1, -2, and –3 and it has been suggested to play roles during the immune response, lymphocyte recirculation, and inflammation. Its role in homing had been suggested by in vitro and in vivo studies but the results were not conclusive enough. Analysis of mice deficient in the αL subunit showed a marked defect in the migration of lymphocytes into peripheral lymph nodes but preserved migration of lymphocytes into mesenteric nodes, Peyer’s patches and the spleen (30). Migration into these sites is abolished in αL null mice by antibodies against α4 or the relevant α4 integrin ligands, confirming a compensatory role for α4 integrins in mediating migration to these sites. Interestingly, analysis of β2 integrin deficient mice has also shown that β2-mediated adhesion is necessary for the emigration of the precursors of dendritic cells into the lung resulting in significantly lower numbers of lung dendritic cells in the mutant mice. The defect is, however, partial, suggesting that other pathways, likely α4 integrins/ VCAM-1, might be involved (31). 6A integrin plays a role in migration of lymphocytes on laminin and likely in homing to peripheral and mesenteric nodes Some integrin subunits display alternative splicing variants. This is the case of α6 integrin subunit whose cytoplasmic domain can be encoded by two alternatively spliced exons. Interestingly, inducible null mice for the variant α6A that can only express α6B show an unsuspected role in lymphocytes. Thus, lymphocytes from α6A null mice demonstrate impaired migration on laminin, and a decreased number of T cells in both peripheral and mesenteric lymph nodes, suggesting a role for the α6A isoform in lymphocyte migration (32). However, experiments injecting α6A null lymphocytes into mouse recipients do not show any defect in homing to these sites and so this point needs to be clarified further. Other integrin subunits such as α5 and αv have been shown not to play a role in homing of leukocytes to immune organs (27). Integrins and migration to inflammatory sites 2 integrins are required for migration of leukocytes to inflammatory sites During the inflammatory response, lymphocytes initiate contact with the activated endothe- 82 VOL. 20 NÚM . 2 / 2001 lium, first by tethering and then by firm adhesion and subsequent transmigration. During this process, the β2 integrin ligand ICAM-1 and the α4 integrin ligand VCAM-1 are up-regulated in the endothelium (26). αL null mice display modest delays in the influx of polymorphonuclear leukocytes and monocytes into the peritoneal cavity in response to an inflammatory stimulus (30). In contrast to expectations, αM null mice do not have any defect in recruitment of neutrophils to sites of inflammation likely due to a compensation by LFA-1 (33,34). However, in αM deficient mice a reduction in neutrophil infiltration is observed in a model of brain ischemia/ reperfusion injury, resulting in a decrease of the damaged area (35). β2 null mice show most of the features of the leukocyte adhesion deficiency (LAD I) seen in humans including leukocytosis, spontaneous infections and impaired emigration of neutrophils into inflamed or infected skin (36,37), and this point will be discussed again later. 4 integrins also play a role in migration of leukocytes to inflammatory sites The role that α4 integrins might have during inflammatory processes had been previously investigated using antibodies against α4 integrins or specific inhibitory peptides (38). These studies had shown a role for α4 integrins during allergic asthma, delayed contact hypersensitivity, experimental allergic encephalomyelitis, rheumatoid arthritis, and chronic inflammatory bowel disease. The analysis of chimeric mice for α4 integrins have demonstrated that in fact α4 integrins participate in vivo in the migration of T lymphocytes to the peritoneal cavity in a model of peritonitis (27). These data reinforce the therapeutic approaches that are under clinical trial using α4 inhibitors for the treatment of different inflammatory diseases always keeping in mind the possible side effects due to the role of α4 integrins in hematopoiesis. 7 integrins are also important for migration of lymphocytes during certain infectious/inflammatory pathologies A model of mice transgenic for an ovalbuminspecific major histocompatibility complex class-I restricted T cell repertoire has been used to investigate the role of β7 integrins during a primary antiviral CD8 T cell response in vivo(39). Analysis of adoptive transfer experiments with β7 null cells from these mice have shown that β7 integrins seem to be critical for migration of activated CD8 T cells to the mesenteric lymph node, Peyer’s patch, and to the small and large intestinal mucosa including INMUNOLOGÍA A. G. ARROYO the epithelium, and for entering of naive CD8 T cells to the lamina propria and intestinal epithelium. However, one of its partners, αE integrin, does not seem to play a role during this primary immune response in vivo. The role of β7 integrins has also been investigated in a model of intestinal inflammation during pathogen-specific protective immunity to enteric helminth infection. The absence of β7 integrins resulted in a delay and reduction of intestinal eosinophilia and mastocytosis during Trichinella spiralis infection of the small intestine, leading to an impaired host protection (40). However, there was no defects in the recruitment of leukocytes after Trichuris muris infection of the large intestine in the β7 deficient mice. Thus, β7 integrins are important for protective immunity against helminth infection of the small intestine. Finally, it has been observed in a model of experimental autoimmune encephalomyelitis that adoptive transfer of either wild-type encephalitogenic T cells to β7-deficient mice, or of β7 null encephalitogenic T cells to wild-type recipients resulted in attenuated forms of the disease indicating that β7 positive cells contribute to disease progression (41). In figure 2, the main pathways of leukocyte homing to lymphoid organs and inflammatory sites demonstrated by the analysis of knockout and chimeric mice are represented. Accordingly to their ligand specificity, integrins (α4β1, α4β7, αEβ7, and β2 integrins) that recognise cellular ligands (VCAM-1, MAdCAM-1, E-cadherin and ICAM-1,-2, and –3, respectively) play a role in recognition of the first tissue barrier i.e. endothe- lium to home to different tissues. On the contrary, integrins such as α5β1 and αvβ1 recognising ECM ligands beneath the endothelium do not seem to play a role. INTEGRINS DURING IN VIVO LEUKOCYTE ACTIVATION Signalling by integrins and other receptors plays an important role in lymphocyte activation (42). Thus, integrin function is regulated by lymphocyte activation. It has also been shown that several integrin ligands, including fibronectin and VCAM-1, can act as co-activation signals for lymphocytes (43). All these data suggest that integrins might participate in lymphocyte activation. This point has been addressed in vivo by the analysis of different mutant mice. 2 integrins act as costimulatory signals for lymphocytes in vivo αLβ2 (LFA-1) had been suggested to play important roles in the induction of immune responses, lymphocyte proliferation, and CTL killing of target cells but the results were controversial. The analysis of mice deficient for either αL or β2 integrins have demonstrated the role of LFA-1 in T cell co-activation by activation with soluble antiCD3, mixed lymphocyte reaction or SEA stimulation, confirming the importance of co-stimulatory signals from β2 integrins in vivo (30,37). Moreover, natural killer cytotoxicity is markedly Peripheral lymph nodes α L, α6A? Skin β2 GUT-associated lymphoid tissue: Mesenteric nodes α4, αL α6A? α4β7 Peyer´s patches αEβ7 Peritoneum β7 Gut (antiviral response, helminth infection) αM, β7 CNS (ischemia/reperfusion, EAE)) Lamina propria Intraepithelium Figure 2. Integrins are important in vivo for establishing the normal pathways of lymphocyte homing and leukocyte migration. In this scheme, we have summarised the integrins that have been demonstrated or suggested (?) to play a role in vivo in the different lymphocyte homing pathways as well as in migration of leukocytes to the inflammatory foci during diverse pathologies. 83 INTEGRINS: WHAT ARE THEY DOING ON LEUKOCYTES IN VIVO? reduced, delayed-type hypersensitivity is absent, and there is no rejection of tumour cells in αL deficient mice suggesting that recirculation of lymphocytes through the lymph node network is impaired. However, CTL activity and secondary antigen restimulation against a systemic infection by virus is normal. It could also be possible that LFA-1 might be differentially required for activation and proliferation, depending on the type of antigen presenting cell involved or the microenvironment of antigen presentation. Altogether, these data demonstrate the role of αLβ2 in TcR-dependent T cell proliferation and in lymphocyte recirculation in vivo. 4, 5, and v integrins are not essential for lymphocyte costimulation in vivo In vitro studies had shown that α4 and α5 integrins might act as co-stimulatory signals for lymphocytes (for review see 43). However, the analysis of the proliferation and activation status of lymphocytes from mice chimeric for α5, αv or α4 integrins have not shown any defect (27). These data suggest that α5, αv, or α4 integrins are not essential for activation of lymphocytes. However, synergism of these adhesion receptors in costimulating lymphocytes under certain conditions in vivo could still be possible. Moreover, because accessory pathways seem to be multiple, it is not possible to rule out replacement by other accessory molecules. Inducible tissue-specific knock-out mice will give further clues about the functions of these receptors during the immune response in vivo. M 2 is critical for neutrophil effector functions in vivo Despite preserved migration of polymorphonuclear neutrophils during inflammation in the absence of αMβ2, neutrophil effector functions are impaired likely because of absent outside-in signalling or deficient signal integration. Thus, αMβ2 has been shown to be important in regulating immune complex-stimulated neutrophil function in vivo resulting in an abrogation of sustained Fcγ receptor-dependent neutrophil adhesion and complement-dependent proteinuria during acute glomerulonephritis in αM deficient mice (44). Moreover, neutrophils deficient in αMβ2 display impaired adherence to hemocyanin coated-glass and fibrinogen coated-filters, iC3bmediated phagocytosis, degranulation, and homotypic aggregation demonstrating the important in vivo role for this β2 integrin in mediating neutrophil effector functions (34). 84 VOL. 20 NÚM. 2 / 2001 INTEGRINS AND LEUKOCYTE APOPTOSIS M 2 regulates neutrophil apoptosis in vivo αM null mice have revealed a completely unsuspected role for this β2 integrin subunit in regulating neutrophil physiology. In response to a chemoattractant stimulus in the peritoneal cavity, these mice show a marked increase in neutrophil accumulation likely due to enhanced neutrophil survival (33). This effect appears to result from the impaired degranulation and phagocytosis, events that normally accelerate apoptosis in neutrophils and that would be regulated by αMβ2 integrin as it has been mentioned earlier (34). 2 integrins might participate in T cell-mediated B cell apoptosis in vivo It has been shown by mAb blocking assays, that interaction of αLβ2 on Th1 T cells with ICAM-1, a ligand for αLβ2, on B cells seems to be essential for Fas-mediated B cell apoptosis. These studies have also been confirmed using B lymphocytes from ICAM-1 deficient mice but it would also be interesting to investigate this point in β2 mutant mice (45). INTEGRIN-DEFICIENT MICE AS IN VIVO MODELS FOR IMMUNE DISEASE 2 null mice as a model of human LAD I disease Leukocyte adhesion deficiency type I (LAD I) is a human disease that results from heterogeneous mutations in the gene encoding the common integrin β2-subunit and it is characterised by delayed separation of the umbilical cord, marked neutrophilia, and recurrent bacterial infections (for review see 46). As it has already been mentioned, partial and complete deficiency for β2 integrins and specific deletions of αLβ2, αMβ2, and αdβ2 (6) have been generated. β2 null mice display most of the features of human leukocyte adhesion deficiency type I (36,37) including neutrophilia and a defect in accumulation of polymorphonuclear neutrophils in inflamed skin. However, migration of neutrophils to other sites such as lung or peritoneum in response to an inflammatory stimulus is preserved suggesting the existence of alternative adhesion mechanisms in mice in contrast to humans in which migration to all inflammatory sites is impaired (46). 6 null mice as a model for asthma and lung inflammation αvβ6 integrin is only expressed in epithelial cells and recognizes fibronectin, tenascin, and INMUNOLOGÍA A. G. ARROYO 85 INTEGRINS: WHAT ARE THEY DOING ON LEUKOCYTES IN VIVO? v i t ronectin. This receptor is barely detected in adult epithelia but it is upregulated upon epithelial injury. Intere s t i n g l y, β6 subunit null mice manifest inflammatory baldness and lung inflammation (47). The skin and the lung lesions with infiltration of macrophages and activated lymphocytes, re s p e c t i v e l y, appear to depend on an e n v i ronmental stimulus. In fact, β6 deficient mice demonstrated airway hyperresponsiveness that resembled asthma. Whenβ6 null mice were given bleomycin in a model of pulmonary fibrosis, they were completely protected from the fibrotic effect (48). Both the exaggerated inflammation and the protection from lung fibro s i s appear to be explained by the novel role recently revealed for this integrin in binding to the latency associated peptide of TGFβ1 and activating extracellular latent TGFβ1 complexes (48). In the absence of β6, there would be less active T G Fβ1 available and it is known that this cytokine is critical for regulation of the immune response. In fact, the defects found in β6 deficient mice partially correlates with the phenotype observed in TGFβ mutant mice (49). VOL. human pathologies such as LAD I, asthma, and lung inflammation and to try novel therapeutic approaches. CO RRESPONDENCE: Alicia G. Arroyo Servicio de Inmunología. Hospital de la Princesa. C/ Diego de León 62 28006 Madrid Tel.: 91 520 23 70 Fax : 91 520 23 74 E-mail : [email protected] References 1. 2. 3. CONCLUDING REMARKS 4. The immune system function relies on a complex network of leukocyte interactions with each other, with the endothelium or with other soluble or ECM ligands. As outlined along this review, the in vivo analysis of mice deficient for diverse members of the integrin superfamily of adhesion receptors has confirmed in many cases and revealed in others the importance that ligand recognition by integrins on the leukocyte surface has for all these processes (Table I). Furthermore, in spite of redundancy of integrin expression and, in some cases, function there are unique and distinct roles for some integrins in leukocyte physiology. Thus, no leukocyte development is possible in the absence of all β1 integrins. Moreover, traffic of leukocytes to lymphoid organs and inflammatory foci depends highly on the presence of integrins on their surface, although other receptors such as selectins can also play a role. During the immune response, the establishment of the immune synapses between T lymphocytes and the APC is critical for activation of the T lymphocytes. The co-stimulatory signals mediated through β2 integrins have been demonstrated to be important in vivo for this proper signalling to occur. Also, αMβ2 is absolutely required for proper effector functions of neutrophils. Finally, other processes important to maintain immune homeostasis such as apoptosis are regulated by αMβ2 on neutrophils and likely by β2 integrins on B lymphocytes. Interestingly, there are also integrin deficient animals that might be used to understand better some 5. 86 20 NÚM . 2 / 2001 6. 7. 8. 9. 10. 11. 12. 13. 14. 15. 16. 17. Dorshkind K. Regulation of hemopoiesis by bone marrow stromal cells and their products. Annu Rev Immunol 1990; 8: 111-37. Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science 1996; 272: 60-6. Hynes RO. Integrins: versatility, modulation, and signaling. Cell 1992; 69: 11-25. Plow EF, Haas TA, Zhang L, Loftus J, Smith JW. Ligand binding to integrins. J Biol Chem 2000; 275: 21785-8. Calderwood DA, Shattil SJ, Ginsberg MH. Integrins and actin filaments: Reciprocal regulation of cell adhesion and signaling. J Biol Chem 2000; 275: 22607-10. Harris ES, McIntyre TM, Prescott SM, Zimmerman GA. The leukocyte integrins. J Biol Chem 2000; 275: 2340912. Hemler ME, Lobb RR. The leukocyte β1 integrins. Curr Opin Hematol 1995; 2: 61-7. Sheppard D. In vivo functions of integrins: lessons from null mutations in mice. Matrix Biol 2000; 19: 203-9. Coulombel L, Auffray I, Gaugler MH, Rosemblatt M. Expression and function of integrins on hematopoietic progenitor cells. Acta Haematol 1997; 97: 13-21. Ryan DH, Nuccie BL, Abboud CN. Inhibition of human bone marrow lymphoid progenitor colonies by antibodies to VLA-4 integrins. J Immunol 1992; 149: 3759-64. Williams DA, Ríos M, Stephens C, Patel VP. Fibronectin and VLA-4 in haematopoietic stem cell-microenvironment interactions. Nature 1991; 352: 438-41. Miyake K, Weissman IL, Greenberger JS, Kincade PW. Evidence for a role of the integrin VLA-4 in lymphohemopoiesis. J Exp Med 1991; 173: 599-607. Hirsch E, Iglesias A, Potocnik AJ, Hartmann U, Fassler R. Impaired migration but not differentiation of hematopoietic stem cells in the absence of β1 integrins. Nature 1996; 380: 171-5. Potocnik AJ, Brakebusch C, Fassler R. Fetal and adult hematopoietic stem cells require β1 integrin function for colonizing fetal liver, spleen, and bone marrow. Immunity 2000; 12: 653-63. Arroyo AG, Yang JT, Rayburn H, Hynes RO. Differential requirements for α4 integrins during fetal and adult hematopoiesis. Cell 1996; 85: 997-1008. Arroyo AG, Yang JT, Rayburn H, Hynes RO. α4 integrins regulate the proliferation/differentiation balance of multilineage hematopoietic progenitors in vivo. Immunity 1999; 11: 555-66. Weissman IL. Developmental switches in the immune system. Cell 1994; 76: 207-18. INMUNOLOGÍA 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. Ogawa M. Differentiation and proliferation of hematopoietic stem cells. Blood 1993; 11: 2844-53. Wagner N, Lohler J, Kumkel EJ, Ley K, Leung E, Krissansen G, et al. Critical role for β7 integrins in formation of the gut-associated lymphoid tissue. Nature 1996; 382: 366-70. Friedrich C, Cybulsky MI, Gutiérrez-Ramos JC. Vascular cell adhesion-molecule-1 expression by hema topoiesis-supporting stromal cells is not essential for lymphoid or myeloid differentiation in vivo or in vitro. Eur J Immunol 1996; 26: 2773-80. Koni PA, Joshi SK, Temann UA, Olson D, Burkly L, Flavell RA. Conditional vascular cell adhesion molecule 1 deletion in mice: Impaired lymphocyte migration to bone marrow. J Exp Med 2001; 193: 741-53. Leuker CE, Labow M, Muller W, Wagner N. Neonatally induced inactivation of the vascular adhesion molecule 1 gene impairs B cell localization and T cell-dependent humoral immune response. J Exp Med 2001; 193: 75567. Taooka Y, Chen J, Yednock T, Sheppard D. The integrin α9β1 mediated adhesion to activated endothelial cells and transendothelial neutrophil migration through interaction with vascular cell adhesion molecule-1. J Cell Biol 1999; 145: 413-20. Grayson, MH, Van der Vieren M, Sterbinsky SA, Gallatin MW, Hoffman PA, Staunton DE, et al. αdβ2 integrin is expressed on human eosinophils and functions as an alternative ligand for vascular cell adhesion molecule 1 (VCAM-1). J Exp Med 1998; 188: 2187-91. Rosenkraz AR, Coxon A, Maurer M, Gurish MF, Austen KF, Friend DS, et al. Impaired mast cell development and innate immunity in Mac-1 (CD11b/CD18, CR3)deficient mice. J Immunol 1998; 161: 6463-7. González-Amaro R, Díaz-González F, Sánchez-Madrid F. Adhesion molecules in inflammatory diseases. Drugs 1998; 56: 977-88. Arroyo AG, Taverna D, Whittaker CA, Strauch UG, Bader BL, Rayburn H, et al. In vivo roles of integrins during leukocyte development and traffic: Insights from the analysis of mice chimeric for α5, αv, and α4 integrins. J Immunol 2000; 165: 4667-75. Schön MP, Arya A, Murphy EA, Adams CM, Strauch UG, Agace WW, et al. Mucosal T lymphocyte numbers are selectively reduced in integrin αE (CD103)-deficient mice. J Immunol 1999; 162: 6641-9. Huleatt JW, Lefrancois L. β2 integrins and ICAM-1 are involved in establishment of the intestinal mucosal T cell compartment. Immunity 1996; 5: 263-73. Schmits R, Kundig TM, Baker DM, Shumaker G, Simard JJL, Duncan G, et al. LFA-1 deficient mice show normal CTL responses to virus but fail to reject immunogenic tumor. J Exp Med 1996; 183: 1415-26. Schneeberger EE, Vu Q, LeBlanc BW, Doerschuk CM. The accumulation of dendritic cells in the lung is impaired in CD18-/-but not in ICAM-1-/-mutant mice. J Immunol 2000; 164: 2472-8. Gimond C, Baudoin C, van der Neut R, Kramer D, Calafat J, Sonnenberg A. Cre-loxP-mediated inactivation of the α6A integrin splice variant in vivo: evidence for a specific functional role of α6A in lymphocyte migration but not in heart development. J Cell Biol 1998; 143: 253-66. Coxon A, Rieu P, Barkalow FJ, Askari S, Sharpe AH, von Andrian UH, et al. A novel role for the β2 integrin A. G. ARROYO 34. 35. 36. 37. 38. 39. 40. 41. 42. 43. 44. 45. 46. 47. 48. 49. CD11b/CD18 in neutrophil apoptosis: A homeostatic mechanism in inflammation. Immunity 1996; 5: 65366. Lu H, Smith CW, Perrard J, Bullard D, Tang L, Shappell SB, et al. LFA-1 is sufficient in mediating neutrophil emigration in Mac-1-deficient mice. J Clin Invest 1997; 99: 1340-50. Soriano SG, Coxon A, Wang YF, Frosch MP, Lipton SA, Hickey PR, et al. Mice deficient in Mac-1 (CD11b/ CD18) are less susceptible to cerebral ischemia/reperfusion injury. Stroke 1999; 30: 134-9. Wilson RW, Ballantyne CM, Smith CW, Montgomery C, Bradley A, O’Brien WE, et al. Gene targeting yields a CD18-mutant mouse for study of inflammation. J Immunol 1993; 151: 1571-8. Scharffetter-Kochanek K, Lu H, Norman K, van Nood N, Muñoz F, Grabbe S, et al. Spontaneous skin ulceration and defective T cell function in CD18 null mice. J Exp Med 1998; 188: 119-31. Lobb RR, Hemler ME. The pathophysiologic role of α4 integrins in vivo. J Clin Invest 1994; 94: 1722-8. Lefrancois L, Parker CM, Olson S, Muller W, Wagner N, Puddington L. The role of β7 integrins in CD8 T cell trafficking during an antiviral immune response. J Exp Med 1999; 189: 1634-8. Artis D, Humphreys NE, Potten CS, Wagner N, Muller W, McDermott JR, et al. β7 integrin-deficient mice: delayed leukocyte recruitment and attenuated protective immunity in the small intestine during enteric helminth infection. Eur J Immunol 2000; 30: 1656-64. Kanwar JR, Harrison JE, Wang D, Leung E, Muller W, Krissansen GW. β7 integrins contribute to demyelinating disease of the central nervous system. J Neuroimmunol 2000; 103: 146-52. Clark EA, Brugge JS. Integrins and signal transduction pathways: the road taken. Science 1995; 268: 233-9. Morimoto C, Iwata S, Tachibana K. VLA-4 mediated signaling. Curr Top Microbiol Immunol 1998; 231: 1-22. Tang T, Rosenkraz A, Assmann KJM, Goodman MJ, Gutiérrez-Ramos J-C, Carroll MC, et al. A role for Mac1 (CD11b/CD18) in immune complex-stimulated neutrophil function in vivo: Mac-1 deficiency abrogates sustained Fcγ receptor-dependent neutrophil adhesion and complement-dependent proteinuria in acute glomerulonephritis. J Exp Med 1997; 186: 1853-63. Wang J, Lenardo MJ. Essential lymphocyte function associated-1 (LFA-1): Intercellular adhesion molecule interactions for T cell-mediated B cell apoptosis by Fas/APO-1/CD95. J Immunol 1997; 186: 1171-6. Etzioni A, Doerschuk CM, Harlan JM. Of Man and Mouse: Leukocyte and endothelial adhesion molecule deficiencies. Blood 1999; 94: 3281-8. Huang X, Wu J, Cass D, Erle D, Corry D, Young S, et al. Inactivation of the integrin β6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J Cell Biol 1996; 133: 921-8. Munger JS, Huang XZ, Kawakatsu H, Griffiths MJD, Dalton SL, Wu JF, et al. The integrin avβ6 binds and activates TGFβ1: a mechanism for regulating pulmonary inflammation and fibrosis. Cell 1999; 96: 319-28. Shull MM, Ormsby I, Kier AB, Pawlowski S, Diebold RJ, Yin M, et al. Targeted disruption of the mouse transforming growth factor-β1 gene results in multifocal inflammatory disease. Nature 1992; 359: 693-9. 87