* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Immunology: The Basics and Beyond
DNA vaccination wikipedia , lookup
Lymphopoiesis wikipedia , lookup
Immune system wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Molecular mimicry wikipedia , lookup
Adaptive immune system wikipedia , lookup
Innate immune system wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
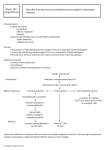
What is Immunology? • Immunology is the study of the body’s defenses against pathogens (viruses, bacteria, fungi, parasites) • Not a well-funded science in the US in the 1970’s and then came AIDS and other diseases that compromise the immune system plus the advent of work on vaccines to treat cancer Immunology: The Basics and Beyond Cindy Wilkening May 20, 2009 Adapted from WWW outline by Dr. J.A. Wise, Dept of Biology, Hampton University 2 Types of Immunity History and Overview • In the late 1700’s, Edward Jenner administered the first vaccination against smallpox • Innate Immunity is present from birth. It is fast but nonspecific. – Macrophages are part of innate immunity and are phagocytic cells that can kill pathogens. Discovered by Elie Metchnikoff in the late 1880’s. – A vaccine is a dead or weakened pathogen used to induce immunity without causing any disease • Adaptive Immunity is acquired during an individual’s lifetime. It is slow but specific. • In the late 1800’s, Louis Pasteur developed cholera and rabies vaccines 3 4 Adaptive Immunity • Antibodies (Ab) or Immunoglobulins (Ig) are substances in the serum of infected individuals that bind pathogen. Discovered in the late 1800’s. • Antigens are generally foreign substances (but can be self) that bind to specific antibodies or cell receptors Macrophages Dendritic Cells 5 6 1 Adaptive Immunity CD molecules • Lymphocytes are required for all forms of adaptive immunity. • Each lymphocyte is specific for one antigen (monospecificity). • A clone is a group of genetically identical lymphocytes specific to a particular antigen. There can be more than 1 clone per antigen. Discovered in the 1960’s. • CD or “cluster of differentiation” are cell membrane molecules that are used to classify cells into subsets • A monoclonal antibody (Mab) can be manufactured from one clone of cells specific to one cell membrane molecule • Mabs are used to define the cluster in vitro • Examples are CD3 on T-cells, CD19 on Bcells, CD34 on stem cells 7 8 Features of Adaptive Immunity Clonal Selection Theory • Clonal Selection and Tolerance • Explains how adaptive immunity works. • Specific lymphocyte clones exist in small numbers before exposure to pathogen. • After exposure, clones specific to the pathogen proliferate, increase in number and differentiate into effector cells, • After fighting off pathogen, there are more cells of these specific clones in the body – Receptor repertoire are the receptors on all lymphocytes that recognize all antigens except self-antigens – Central tolerance is the elimination of lymphocytes with receptors for self antigens early in development of the lymphocytes 9 • Immunological memory happens when the body is re-exposed to pathogen. The adaptive immunity is faster and stronger because of the increased number of pathogen-specific lymphocytes 10 11 12 Lymphocyte Differentiation • B cells (as plasma cells) secrete antibody • T cells develop into effector cells that either kill pathogen-infected cells or activate other parts of the immune system • How do these cells originate? From hematopoietic stem cells in the bone marrow 2 13 14 15 16 Adult Normal White Cell Counts in Blood and 5-Part Differential • B-CELLS MATURE IN BONE MARROW • T-CELLS START IN BONE MARROW AND FINISH MATURING IN THYMUS. • White blood cells: 5000 to 9000 cells per mm3 • • • • • Neutrophils: 55.0% Lymphocytes: 35.0% Monocytes: 6.5% Eosinophils: 3.0% Basophils: 0.5% • T-CELLS AND B-CELLS THAT HAVE NOT YET BEEN EXPOSED TO ANTIGEN ARE CALLED NAÏVE LYMPHOCYTES (3000 to 7000/mm3) (1000 to 4000/mm3) (100 to 600/mm3) (50 to 250/mm3) (25 to 100/mm3) • ANTIGEN TRAVELS IN BLOOD TO THE SPLEEN AND LYMPH TO THE LYMPH NODES WHERE IT IS FILTERED OUT AND ACTED UPON BY LYMPHOCYTES • DISTINCT AREAS FOR B-CELLS AND T-CELLS IN THE LYMPHOID TISSUES 17 18 3 Antigen Receptors on Lymphocytes • Membrane-bound Immunoglobulin (Ig) on B-cells • T-cell receptor on T-cells B-CELL RECEPTOR 19 T-CELL RECEPTOR 20 Immune Response to Pathogens • Lymphocyte activation 21 – B-cells: first signal is binding of antigen to surface Ig, second signal provided by T-cells via cytokines which are like localized hormones – T-cells: first signal is binding of antigen to Tcell receptor, second signal by antigenpresenting cells which are macrophages or dendritic cells – Activated lymphocytes proliferate (clonal expansion) then differentiate into effector cells 22 Major Histocompatibility Complex (MHC) • T-lymphocyte cells recognize antigen bound to MHC molecules • MHC Class I bearing cells (most nucleated cells) interact with CD8 positive cytotoxic T-lymphocyte cells that act by direct cell killling • MHC Class II bearing cells (antigen presenting cells) interact with CD4 positive helper T-lymphocyte cells which activate other T-lymphocyte and B-lymphocyte responses 23 24 4 HLA are Human MHC Molecules • HLA are human leukocyte antigens • Each person has six class I MHC-alleles (1 of HLA-A, -B, and -C from each parent) • Each person has at least 6 class II MHCalleles (1 of HLA-DQ and -DP, and 1 or 2 of -DR) • Many different alleles in population for each HLA type 25 26 Humoral Immunity Cell-Mediated Immunity • Mediated by Ig antibodies (IgM, IgA, IgD, IgE, and IgG) • Antibodies released into blood from plasma cells (an effector B-cell) • Good against extracellular pathogens and toxins (eg from bacteria) • Neutralization is physical binding by antibody • Opsonization is binding of antibody to pathogen which causes antigen to be ingested more rapidly by macrophages • Activation of complement can lyse and kill bacteria 27 • Carried out by T-cells, macrophages, and other cells and is good against cells with intracellular pathogens • Helper T cells activate other cells of the immune system; the ‘Generals’ • Cytotoxic T cells can destroy cells infected with viruses by direct killing with perforin and granzymes • Phagocytes digest cell fragments from killed cells 28 MEMORY CELLS PRIME IMMUNE SYSTEM 29 30 5 Tumor Cell Escape Types of tumor antigens • Mutated self protein • Product of oncogene or mutated tumor suppressor gene • Overexpressed or aberrantly expressed self protein • Oncogenic virus • Failure to produce or loss of tumor antigen • Mutations in MHC genes or genes needed for antigen processing • Production of immunosuppressive proteins or expression of inhibitory cell surface proteins • Since many tumors arise from self cells, the tumor may be ignored as self tissue 31 Types of Cancer Vaccines 32 Types of Cancer Vaccines • Viral vectors and DNA vaccines • Antigen/adjuvant vaccines – Cancer cell antigen plus adjuvant (a substance that causes an immune response). Expected that immune system will respond to tumor cells that express the vaccine antigen • Whole cell tumor vaccines – Cells taken from patients own tumor (autologous) or from other patients (allogeneic). Expect to stimulate an immune response – DNA with gene for cancer antigen is manipulated in the lab so it is taken up by antigen-presenting cells (APC). The antigen is displayed on APC cell surface. Expected that immune system will attack tumor cells with this antigen (Not all DNA vaccines use viral vectors) • Idiotype vaccines • Dendritic cell (DC) vaccines – DC removed from patient’s blood and then stimulated with patient’s own cancer antigens, grown in petri dishes then re-injected into the patient. Expected to activate T-lymphocytes to multipy and attack tumor cells with DC antigen – Antibodies contain proteins and carbohydrates which can be antigens. Cancer antibodies (called idiotype antibodies) are unique for each person and can be used to trigger an immune response in a way similar to antigen vaccines 33 Treatment Vaccines Under Investigation • • • • • • • • • Patient-specific vaccines Prostate Specific Antigen (PSA) Sialyl Tn (STn) Heat Shock Proteins (HSPs) Ganglioside molecules Carcinoembryonic antigen (CEA) MART-1 (or Melan-A) Tyrosinase General DNA vaccines to modify genetic structure of cancer cells 34 Approved Preventative Vaccines • Hepatitis B – Prevents infection with hepatitis B virus associated with some forms of liver cancer • Gardasil – Prevents infection from 2 types of human papillomavirus (HVP) which causes 70 percent of cervical cancer worldwide 35 36 6 HLA/MHC and Transplantation HLA/MHC and Transplantation • During maturation in thymus, CD8+ and CD4+ T cells selected to recognize peptides displayed by self MHC molecules • Transplantation can include blood, cells, tissues from source other than self (allogeneic) • T-cells from self cross-react with non-self MHC molecules and attack the non-self cells • Need for tissue typing to match HLA molecules from donor to those of transplant host • Need to use immunosuppressant drugs such as cyclosporine or steroids to suppress immune reaction in host • If mature T-cells of donor are transplanted, they can attack recipient’s tissues leading to serious clinical reaction called Graft vs Host disease. This may happen especially in bone marrow or stem cell transplants. 37 38 Some Methods of Assessing the Immune System HLA/MHC and Transplantation • There is also Graft vs Leukemia where it is beneficial if the donor cells kill off the receipient bad immune cells. There is a slight mismatch made in the HLA typing for this to happen. • Flow cytometry – – – – Lymphocyte subsets Tetramer Assay Cytokine Assay CFSE Flow-based Cell Proliferation Assay • Serial dilution assays – Natural Killer Cell (NK) assay as an example • Delayed type hypersensitivity (DTH) test • ELISA • ELISPOT 39 40 Flow Cytometry Sample Prep for Subsets Flow cytometry “A flow cytometer is an instrument that illuminates cells as they flow individually in front of a light source and then detects and correlates the signals from these cells that result from the illumination.” Givan, 2004 A flow cytometer can also deflect cells based on the laser illumination and sort them on size or fluorescence 41 • Whole blood collected from patients into tubes with anticoagulant • Red cells are lysed with a lysing agent • A monoclonal antibody (Mab) is connected to a fluorochrome and this complex is added to the tube. Fluorochromes emit fluorscence upon excitation at a specific wavelength • 1 to as many as 17 Mab- fluorochromes can be added to tube depending on number of collectors on machine 42 7 43 44 DNA CONTENT 45 S-PHASE FRACTION G0/G1 is resting/active cell phase S-phase is DNA synthesis G2/M 2x DNA content for mitosis 46 Tetramer Assay • Advantages – Specific, sensitive, can assess complex cell phenotypes by flow – Can detect dysfunctional cells which arise during chronic exposure to antigens such as tumors or HIV virus • Disadvantages – Only single specificities can be analyzed – Measure intensity but not breadth of response 47 48 8 What is a tetramer? • Complex of 4 MHC molecules bound with peptide of interest and labeled with fluorochromes • Purchase tetramers from a company or from NIH for custom tetramers • Use as a stain in a flow cytometric assay • Often a “rare event” (much less than 1%), need 105 to 106 lymphocytes (events) to detect 49 TETRAMER RESULTS 50 Tetramer Assay • Results are frequency of tetramer positive cells reported as a fraction of a major lineage subset used to define them such as CD3+CD8+ • Can also be reported as number of tetramer positive cells per volume of blood if beads are used on the flow cytometer 51 52 Cytokine Flow Cytometry Cytokine Flow Cytometry • Antigen-stimulated CD4+ and CD8+ Tcells can be detected by directly measuring internal cytoplasmic levels of cytokines such as IFN-gamma and IL2 by flow cytometry • PBMCs or cell suspensions are stimulated in vitro for a short time with antigens that cause cytokines to be made in the cell cytoplasm. • Drugs that block cytokine synthesis are then added to stop cytokine production • Cell membranes are permeabilized with mild detergent to allow fluorescent tagged cytokine-specific Mabs to get into cell • Cells can be stained with Mabs for lineage markers such as CD3, CD4, CD8 and for activation markers such as CD69 • Results are “rare events” and require up to 106 events to detect • Results can be expressed as percents 53 54 9 CFSE Flow-based Proliferation Assay CFSE Flow-based Proliferation Assay • CFSE is carboxyfluorescein diacetate succinimidyl ester • Labels long-lived intracellular molecules with highly fluorescent dye • After labeling with CFSE, cells are stimulated with antigen or mitogen of choice • Following each cell division, progeny have half of the fluorescence as parent cell • Can detect up to 8 to 10 cell divisions • Track cells from Day 2 to Day 14 after CFSE 55 • In addition to CFSE, Mabs to surface molecules on the lymphocytes can determine how phenotypic properties change with cell division • CFSE-labeled cells can be sorted on a flow cytometer and analyzed in functional assays such as secretion of cytokines or antibodies • With use of beads, final data are number of cells per total cells within a cell culture for each division number 56 NK Cell Assay • • • • NK cells part of innate immune system Not antigen dependent Can kill target cells such as K562 cell line Radioactive Chromium (51Cr) is added to K562 medium and is taken up by cells • K562 cells are washed to remove excess 51Cr 57 58 NK Cell Assay NK cell assay • 96-well plate is prepared with either whole blood or PBMCs of patient • Add dilutions of labeled K562 cells to wells containing PBMCs to yield specific effector: target ratios from 50:1 to 6.25:1 • Also have wells with labeled K562 cells but no PBMCs for spontaneous release 59 • Also have wells with K562 cells only to which a detergent is added to disrupt cell membranes and get maximum release of 51Cr • Incubate for set time, stop incubation with cold medium, remove supernatant and transfer to counting vials • Count on a gamma counter to get counts per minute (CPM) which is used to calculate % specific lysis 60 10 Delayed Type Hypersensitivity Test NK Cell Assay • % specific lysis=100* (mean experimental cpm-mean spontaneous release cpm) (mean max release cpm-mean spontaneous release cpm) • #Lytic Units (LU) per 107 effector cells= #Effector cells (E:T)(#Target cells) 166 Lytic Units per 107 effector cells. 105 or 106 effector cells can also be used • Measure of intact cell-mediated immunity • Use antigens that patient has been exposed to such as Candida or Tetanus • Inject small amount of antigen suspended in fluid just under skin of lower arm • After 48 to 72 hours, check for induration at site of injection • Measure largest diameter • Diameter >=5mm indicates intact CMI 61 DTH Test • If it is known that the individual has been exposed to the antigen but there is no induration, called anergy • Abnormal DTH can be a sign of frequent infections, autoimmunity, and malignancy • DTH also used to help diagnose infections such as TB • DTH used to aid in cell-mediated immune response to vaccines 62 Elisa Assay (Enzyme-linked immunosorbant assay) Antigen on Solid Phase 63 Elisa Assay: Antibody on Solid Phase 64 Elisa Assay • Color of fluid in wells is measured in a reader and expressed as relative light units • A standard curve is generated with known amounts of antibody and its relative light units • Final results expressed as pg/ml concentration of cytokine, for example 65 66 11 ELISPOT Assay • ELISPOT Assay is Enzyme-Linked Immunospot Assay • Detection of secreted molecule at site of secreting cells • 100 to 400 fold more sensitive than ELISA • Final results expressed as Spot Forming Cells (SFC) per 106 cells 67 68 69 70 71 72 12 Book Resources Journal Resources • Basic Immunology (3rd edition) by AK Abbas and AH Lichtman, 2008 • Flow Cytometry, Clinics in Laboratory Medicine, 2007 • Manual of Molecular and Clinical Laboratory Immunology (7th edition) by B Detrick et al,2006 • Cellular and Molecular Immunology (3rd edtion) by AK Abbas et al, 1997 • Immunobiology (5th edition) by Janeway et al, 2001 • Flow Cytometry: An Introduction by AL Givan in Flow Cytometry Protocols (2nd edition) by TS Hawley and RG Hawley, 2004 • Flow Cytometry: First Principles (2nd edition) by AL Givan, 2001 • Schaum’s Outline of Theory and Problems of Immunology by G Pinchuk, 2002 • A Manual of Laboratory and Diagnostic Tests (5th edition) by F Fischbach, 1996 • Immunology at a Glance (8th edition) by JHL Playfair and BM Chain, 2005 • How the Immune System Works (3rd edition) by L Sompayrac, 2008 • Guinn, B-A et al, Recent advances and current challenges in tumor immunology and immunotherapy. Molecular Therapy, 2007, vol 15, p 1065-1071 • Altman, JD, Flow cytometry applications of MHC tetramers, Methods in Cell Biology, 2004, vol 75, p 433-452 • Lyons, AB et al. Flow cytometric analysis of cell division history using dilution of carboxyfluorescein diacetate succinimidyl ester, a stably integrated fluorescent probe. Methods in Cell Biology, 2001, vol 63, p 375-398 • Klenerman, P et al, Tracking T-cells with tetramers: new tales from new tools. Nature Reviews Immunology, 2002, vol 2, p 263-272 • Walker, EB et al. Monitoring immune responses in cancer patients receiving tumor vaccines. Intern. Rev. Immunol, 2003, vol 22, p 283319 • Quah, BJC et al. Monitoring lymphocyte proliferation in vitro and in vivo with the intracellular fluorescent dye CFSE, Nature Protocols, 2007, vol 2, p 2049-2056 73 74 WWW Resources • www.utpa.edu/faculty/materon/3403/immunopaper.html (now defunct; was JA Wise outline) • www.cancer.gov/cancertopics/factsheet/cancervaccine • www.cancer.gov/clinicaltrials/learning/cancervaccines • www.elispot-analyzers.de/english/elispot-animation.html • www.ask.com/pictures?q=immune+system&qsrc=0&o=0&l=dir • www.freewebs.com/immunology/elispot-wells.html 75 13