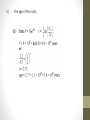* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Unit 7 Review
Nuclear fusion–fission hybrid wikipedia , lookup
Technetium-99m wikipedia , lookup
Isotopic labeling wikipedia , lookup
Nuclear fission product wikipedia , lookup
Nuclear fission wikipedia , lookup
Nuclear fusion wikipedia , lookup
Radioactive decay wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Valley of stability wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Unit 7 Review Quiz #2 Solutions 1. A nucleus of the nuclide 19K (potassium-40) decays to a stable nucleus of the nuclide 18Ar (argon-40). State the names of the two particles emitted in this decay. Since we lost a proton in the process, it is a positron emission b+, it emits a positron (e+) and a neutrino 2. One of the wavelengths in the absorption spectrum of helium occurs at 588 nm. Show that the energy of a photon of wavelength 588 nm is 3.38 × 10 –19 J. E hc 6.62 10 3 10 34 588 10 9 1.99 10 25 588 10 9 3.38 10 19 J 8 3. The nucleus 30 P undergoes radioactive decay to the nucleus 15 The particles emitted in the decay are A. a positron and an antineutrino. B. an electron and an antineutrino. C. a positron and a neutrino. D. an electron and a neutrino 30 Si. 14 4. This question is about nuclear reactions. (a)(i) Distinguish between fission and radioactive decay. Fission: nucleus splits; into two parts of similar mass; radioactive decay: nucleus emits; a particle of small mass and / or a photon; A nucleus of uranium-235 ( 235 U) may absorb a neutron and 92 then undergo fission to produce nuclei of strontium-90 ( 90 Sr) 38 and xenon-142 ( 14254 Xe ) and some neutrons. The strontium-90 and the xenon-142 nuclei both undergo radioactive decay with the emission of β– particles. (ii) Write down the nuclear equation for this fission reaction. (iii) State the effect, if any, on the mass number (nucleon number) and on the atomic number (proton number) of a nucleus when the nucleus undergoes β– decay. Mass number: mass number unchanged Atomic number: atomic number increases by +1 The uranium-235 nucleus is stationary at the time that the fission reaction occurs. In this fission reaction, 198 MeV of energy is released. Of this total energy, 102 MeV and 65 MeV are the kinetic energies of the strontium-90 and xenon-142 nuclei respectively. (b)(i) Suggest what has happened to the remaining 31 MeV of energy. kinetic energy of neutrons; and energy of gamma ray photons; 5. This question is about the Bohr model of the hydrogen atom. (a) The diagram below shows the three lowest energy levels of a hydrogen atom as predicted by the Bohr model. Energy n=3 –1.51 eV n=2 –3.40 eV n=1 –13.6 eV State two physical processes by which an electron in the ground state energy level can move to a higher energy level state. collisions with (external) particles; heating the gas to a high temperature; absorption of photons; (b) A parallel beam of white light is directed through monatomic hydrogen gas as shown in the diagram below. The transmitted light is analyzed. hc White light consists of photons E that range in wavelength from 6.62 10 34 3 108 approximately 400 nm for violet 658 10 9 to 700 nm for red light. 1.99 10 25 Determine that the energy of photons 658 10 9 of light of wavelength 658 nm is 3.02 10 19 J about 1.89 eV. 19 white light beam hydrogen gas transmitted light beam 3.02 10 J 1.89eV 19 1.6 10 J / eV 7. This question is about radioactive decay. A nucleus of the isotope xenon, Xe-131, is produced when a nucleus of the radioactive isotope iodine I-13 decays. (a) Explain the term isotopes. the nuclei of different isotopes of an element have the same number of protons; but different numbers of neutrons; (b) Fill in the boxes below in order to complete the nuclear reaction equation for this decay. 53 The activity A of a freshly prepared sample of the iodine isotope is 6.4 × 105 Bq and its half-life is 8.0 days. 8. This question is about radioactive decay and the age of rocks. A nucleus of the radioactive isotope potassium-40 decays into a stable nucleus of argon-40. (a) Complete the equation below for the decay of a potassium-40 nucleus. A certain sample of rocks contains 1.2 × 10–6 g of potassium-40 and 7.0 × 10–6 g of trapped argon-40 gas. (b) Assuming that all the argon originated from the decay of potassium-40 and that none has escaped from the rocks, calculate what mass of potassium was present when the rocks were first formed. 6 6 6 1.2 10 7 10 8.2 10 g The half-life of potassium-40 is 1.3 × 109 years. (c) Determine (i) the decay constant of potassium-40; ii) the age of the rocks. 6. Which of the following graphs best shows how photon energy E varies with the wavelength λ of the light? A. 0 C. B. E 0 0 D. E 0 E E 0 0 E E 0 hc 0 1.99 10 25 Jm 9. Nuclear binding energy and nuclear decay (a) State what is meant by a nucleon. (a nucleon is either) a proton or a neutron (b) Define what is meant by the binding energy of a nucleus. energy released when a nucleus is formed from its constituent nucleons / (minimum) energy needed to break a nucleus up into its constituent nucleons Use the graph to explain why energy can be released in both the fission and the fusion processes energy release being possible as products have higher (average) binding energy per nucleon; A sample of carbon-11 has an initial mass of 4.0 x 10–15 kg. Carbon-11 has a half-life of approximately 20 minutes. Calculate the mass of carbon-11 remaining after one hour has elapsed. realization that time elapsed is 3 half-lives; so one eighth remains 5 x 10-16 kg; 1hr. 3 20 min . 3 1 1 2 8 1 4.0 10 15 5 10 16 8 (e) Uranium-238, undergoes a-decay to form an isotope of thorium. Write down the nuclear equation for this decay. 10. When the isotope aluminium-27 is bombarded with alpha particles, the following nuclear reaction can take place Which one of the following correctly gives the atomic (proton) number and mass (nucleon) number of the nucleus X? 11. The initial activity of a sample of a radioactive isotope of half-life 10 hours is A. What is the age of the sample when its activity is 32A ? x A. 30 hours 1 1 2 32 B. 40 hours 1 C. 50 hours log 32 5 x D. 320 hours 1 log 2 5 10 50hrs 12. The binding energy per nucleon of the nucleus is 73 Li approximately 5 MeV. The total energy required to completely separate the nucleons of this nucleus is approximately A. 15 MeV. B. 20 MeV. C. 35 MeV. 7 nucleons 7 x 5 = 35 MeV D. 50 MeV. This question is about nuclear binding energy. The table below gives the mass defect per nucleon of deuterium 21 H and helium-4 42 He. Explain the term mass defect the difference in mass between mass of nucleus; and mass of (totally) separate nucleons; Calculate the energy, in joule, that is released when two deuterium nuclei fuse to form a helium-4 nucleus. mass of helium-4 = 4 x 0.00760 = 0.0304 u and mass of two deuteriums = 4 x 0.00120 = 0.0048u; mass defect = 0.0256u; energy = 0.0256 x 1.66 x 10-27 x (3 x 108)2; = 3.8 x 10-12 J;