* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Lecture 2: Glycogen metabolism (Chapter 15)
Adenosine triphosphate wikipedia , lookup
Enzyme inhibitor wikipedia , lookup
Metalloprotein wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Citric acid cycle wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Biosynthesis wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
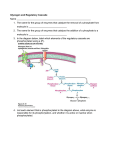
Lecture 2: Glycogen metabolism (Chapter 15) First…. Fig. 15.1 Review: Animals use glycogen for ENERGY STORAGE. Glycogen is a highly-branched polymer of glucose units: Basic structure is similar to that of amylopectin, but with only about 8 to 12 glucose units between branch points (n = 4 to 6). GLYCOGEN BREAKDOWN INSIDE CELLS: Glycogen's glucose units are mobilized by their sequential removal from the glucan chain's nonreducing ends — that is, the ends that lack a C1-OH group. This is the reducing end of glucose: Fig. 15-2a The ends of some sugars have a free anomeric carbon, which can act as a mild reducing agent. (In glycogen, however, the reducing end is actually bound by a protein named GLYCOGENIN.) The branched structure of glycogen permits the rapid release of glucose simultaneously from every non-reducing end of every branch: (These red arrows point to the nonreducing ends.) Fig. 15-2b (modified) Only ONE reducing end per molecule (Note that the number of glucose units between branch points in this figure is not accurate. Don’t let this confuse you!) Reminder: The reducing end is bound by GLYCOGENIN GLYCOGENIN Fig. 15-2b (modified) Why use glycogen to store energy rather than just using fat? (Since fat is more abundant than glycogen in the body and also stores energy) 1. Muscles "mobilize" (i.e., convert to energy) glycogen faster than fat. 2. Fatty acid residues cannot be metabolized anaerobically (that is, without oxygen). (If you want to burn fat while you are exercising, you must be able to breathe fairly easily.) 3. Animals cannot convert fat to glucose, so fat metabolism cannot maintain blood glucose levels. (Glucose is ”brain food"— it is the major energy form that crosses the blood-brain barrier.) Glycogenolysis (or glycogen breakdown) requires 3 major enzymes: 1) GLYCOGEN PHOSPHORYLASE (Fig. 15-4; more later) 2) GLYCOGEN DEBRANCHING ENZYME (Fig. 15-6) 3) PHOSPHOGLUCOMUTASE (Fig. 15-7): Glycogenolysis requires 3 major enzymes: 1) GLYCOGEN PHOSPHORYLASE (or simply PHOSPHORYLASE) See Fig. 15-4 (next slide) for GP’s reaction mechanism. Note that GP catalyzes bond cleavage by PHOSPHOROLYSIS, as opposed to HYDROLYSIS. The overall reaction is: Glycogen(n residues) + Pi inorganic phosphate Glycogen (n-1) + G-1-P Glucose-1-phosphate GLYCOGEN PHOSPHORYLASE MECHANISM: Fig. 15-4: Phosphorylase has a “random sequential”enzyme mechanism that involves PLP (pyridoxyl-5’phosphate), a vitamin B6 derivative: NOTE: Phosphorylase only releases units that are 5 or more from the branch point, leaving a “LIMIT BRANCH”…. Glycogenolysis requires 3 major enzymes: 2) GLYCOGEN DEBRANCHING ENZYME (Fig. 15-6) GDE has two enzymatic activities: A) A “debranching” transglycosylase activity B) An hydrolysis activity A B Glycogenolysis requires 3 major enzymes: 3) PHOSPHOGLUCOMUTASE reaction: G-1-P G-1,6-P G-6-P Glucose-1,6-bisphosphate Glucose-6-phosphate Fig. 15-7: Phosphoglucomutase Mechanism (Note that this reaction is fully reversible.) Fig. 15-1: G-6-P is a major intermediate in glucose metabolism Glucose-6-phosphatase hydrolyzes G-6-P to Glucose + Pi in LIVER Important in nucleotide synthesis Fig. 15-1: G-6-P is a major intermediate in glucose metabolism Brief overview next... Glycogen SYNTHESIS requires 3 major enzymes, and occurs by a SEPARATE PATHWAY from glycogenolysis: 1) UDP-GLUCOSE PYROPHOSPHORYLASE (Fig. 15-9): G-1-P + UTP Uridine triphosphate UDP-glucose (UDPG) + Uridine diphosphate glucose PPi inorganic pyrophosphate 2) GLYCOGEN SYNTHASE (Fig. 15-10): UDPG + Glycogen(n units) UDP + Glycogen(n+1 units) This reaction must be “primed” by GLYCOGENIN 3) GLYCOGEN BRANCHING ENZYME (Fig. 15-11) or AMYLO (1,4 1,6) TRANSGLYCOSYLASE. GENERAL RULES FROM ABOVE: BIOSYNTHETIC AND DEGRADATIVE PATHWAYS OF METABOLISM ARE (ALMOST) ALWAYS COMPLETELY DIFFERENT. THAT IS, THEY USED DIFFERENT ENZYMES. POLYMERIZATION OF MONOMERIC UNITS INTO MACROMOLECULES USUALLY REQUIRES A ‘PRIMER’ TO INITIATE THE REACTION. THAT IS, THE FIRST TWO UNITS CANNOT BE LINKED BY THE ENZYME THAT DOES THE POLYMERIZATION. 1. GLYCOGEN PHOSPHORYLASE (or simply PHOSPHORYLASE) • Removes GLUCOSE UNITS from the NONREDUCING ends of GLYCOGEN. • Is a FAST enzyme: the outermost branches of glycogen are degraded in seconds in muscle tissue. • Is a dimer of identical 842-residue subunits (Fig. 15-3). 1. GLYCOGEN PHOSPHORYLASE (continued) • Catalyzes the CONTROLLING STEP in glycogen breakdown. • The standard-state free-energy change ( G°') for phosphorylase reactions is + 3.1 kJ/mol, but the intracellular [Pi] / [G1P] ratio is about 100, so G in vivo is actually about - 6 kJ/mol. 1. GLYCOGEN PHOSPHORYLASE (continued) • It is a highly and complexly regulated enzyme, both by: • ALLOSTERIC INTERACTIONS (Fig. 15-13) — ATP, G6P & glucose inhibit it; AMP activates it and • COVALENT MODIFICATION by phosphorylation and dephosphorylation (Fig. 15-5). Yields 2 major forms of phosphorylase — Phosphorylase A: Has a phosphoryl group esterified to Ser-14 in each subunit (more active) Phosphorylase B: Is not phosphorylated (less active) Look at Kinemages Exercise 14 on the CD with VVP textbook! 1. GLYCOGEN PHOSPHORYLASE (continued) • Only releases units that are 5 or more from the branch. WHY? 1. GLYCOGEN PHOSPHORYLASE (continued) • Only releases units that are 5 or more from the branch. WHY? Robert Fletterick (www.ucsf.edu/pibs/faculty/fletterick.html) solved the 3D structure of Phosphorylase A: Its crevice can admit 4 or 5sugar residues, but it is too narrow to admit a branch. Fig. 15-1: G-6-P is a major intermediate in glucose metabolism NEXT... Glycogen SYNTHESIS requires 3 major enzymes, and occurs by a SEPARATE PATHWAY from glycogenolysis: 1) UDP-GLUCOSE PYROPHOSPHORYLASE (Fig. 15-9): G-1-P + UTP Uridine triphosphate UDP-glucose (UDPG) + Uridine diphosphate glucose PPi inorganic pyrophosphate 2) GLYCOGEN SYNTHASE (Fig. 15-10): UDPG + Glycogen(n units) UDP + Glycogen(n+1 units) This reaction must be “primed” by GLYCOGENIN 3) GLYCOGEN BRANCHING ENZYME (Fig. 15-11) or AMYLO (1,4 1,6) TRANSGLYCOSYLASE. 1) UDP-GLUCOSE PYROPHOSPHORYLASE (Fig. 15-9): G-1-P + UTP Uridine triphosphate UDP-glucose (UDPG) + Uridine diphosphate glucose PPi inorganic pyrophosphate The G°’ of this reaction is nearly ZERO, but the PPi formed is hydrolyzed to 2 Pi (orthophosphate) in a highly EXERGONIC reaction the the omnipresent enzyme, INORGANIC PYROPHOSPHATASE. Therefore, the overall reaction is also highly exergonic: G°’ (kJ/mol) GIP + UTP UDPG + PPi ~0 H2O + PPi 2 Pi - 33.5 GIP + UTP UDPG + 2 Pi - 33.5 OVERALL UDPG is a HIGH ENERGY compound that can donate GLYCOSYL units to the growing glycogen chain. No further energy is required for glycogen synthesis. IMPORTANT GENERAL NOTE: The cleavage of a nucleoside triphosphate (NTP) to form PPi is a common synthetic strategy. The free energy of PPi hydrolysis (by inorganic pyrophosphatase) can be utilized together with the free energy of NTP hydrolysis to drive an otherwise endergonic reaction to completion. (We will see this over and over and over this semester!) 2) GLYCOGEN SYNTHASE MECHANISM (Fig. 15-10): UDPG + Glycogen(n units) UDP + Glycogen(n+1 units) The glycosyl unit of UDPG is transferred to the C(4)-OH on one of the non-reducing ends of glycogen, forming an (1 4) glycosidic bond. Note that this step makes -amylose, not the branched structure of glycogen. The ∆G°’ for this reaction is -13.7 kJ/mol, making this reaction spontaneous (exergonic) under the same conditions that glycogen breakdown is exergonic. Therefore, the rates of the two reactions must be independently and tightly controlled. For each molecule of GIP that is converted to glycogen, one molecule of UTP is hydrolyzed to UDP + Pi. The UTP is replenished by the enzyme NUCLEOSIDE DIPHOSPHATE KINASE: UDP + ATP UTP + ADP (UTP hydrolysis is energetically equivalent to ATP hydrolysis.) GLYGOGENIN and Glycogen “Priming” Glycogen synthesis can only occur by extending an already existing (1 4)-linked glucan chain. Therefore, how can it get started in the first place? Answer: The first step in glycogen synthesis is the attachment of a glucose residue to the -OH group on Tyr-194 of GLYCOGENIN. This attachment step is done by the enzyme TYROSINE GLUCOSYLTRANSFERASE. Glycogenin then autocatalytically extends the glucan chain by up to 7 residues long (also donated by UDPG). Glycogen synthase can then attach glucose residues to this glycogen “primer”. Each molecule of glycogen is associated with ONE molecule each of glycogenin and glycogen synthase. 3) GLYCOGEN BRANCHING ENZYME (Fig. 15-11) or AMYLO (1,4 1,6) TRANSGLYCOSYLASE: Breaks (1 4) glycosidic bonds and forms (1 6) linkages. Transfers terminal chain segments of about 7 residues to the C(6)-OH groups of glucose residues. Each transferred segment must come from a chain of at least 11 residues, and the attachment point must be at least 4 residues away from another branch point. Segment can be moved to the same or a different chain. Note: Not to be confused with Glycogen Debranching Enzyme! Control of glycogen metabolism is very complex. It involves: • allosteric regulation of both GS & GP • substrate cycles • enzyme-catalyzed covalent modification of both GS &GP • covalent modifications are under hormonal control in the body, through their own enzymatic cascades In LIVER: Glycogen metabolism is ultimately controlled by GLUCAGON — a 29 amino acid-long polypeptide hormone that is secreted from the pancreas into the bloodstream (liver cells have glucagon receptors). In MUSCLES (and various other tissues): Is controlled by the adrenal hormones EPINEPHRINE (adrenalin) and NOREPINEPHRINE (noradrenalin). These hormones act at cell surfaces to stimulate ADENYLATE CYCLASE, thus increasing [cAMP]. cAMP acts inside cells as a ‘second messenger’ for the hormones. Cells have many cAMP-dependent PROTEIN KINASES whose activities increase upon cAMP binding. (Reminder: Kinases catalyze the transfer of phosphoryl groups between ATP and other molecules, proteins in this case.) Liver maintains blood [glucose] at ~5 mM; if it drops to half of this, a coma results. Upon blood [glucose] decrease, the liver releases glucose to the blood; glucose triggers pancreas to release glucagon, which causes increase [cAMP] in liver, which in turn stimulates glycogen breakdown. Glucose diffuses freely out of liver cells, causing an increase in blood [glucose]. High blood [glucose] causes release of INSULIN from the pancreas to the blood. The rate of glucose TRANSPORT across many cell membranes increases in response to insulin.