* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Louie de Broglie
Density functional theory wikipedia , lookup
Particle in a box wikipedia , lookup
Elementary particle wikipedia , lookup
Dirac equation wikipedia , lookup
Ferromagnetism wikipedia , lookup
Bremsstrahlung wikipedia , lookup
Molecular Hamiltonian wikipedia , lookup
Double-slit experiment wikipedia , lookup
Relativistic quantum mechanics wikipedia , lookup
Chemical bond wikipedia , lookup
X-ray fluorescence wikipedia , lookup
Molecular orbital wikipedia , lookup
Quantum electrodynamics wikipedia , lookup
Matter wave wikipedia , lookup
Auger electron spectroscopy wikipedia , lookup
Wave–particle duality wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Tight binding wikipedia , lookup
Theoretical and experimental justification for the Schrödinger equation wikipedia , lookup
Hydrogen atom wikipedia , lookup
Electron-beam lithography wikipedia , lookup
Atomic theory wikipedia , lookup
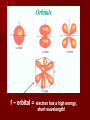
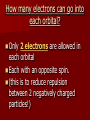
The Quantum Model of the Atom CP Chemistry Louie de Broglie Proposed that all particles of matter that move exhibit wave like behavior (even a baseball!) He came up with the following equation that relates the wavelength of a particle to its mass and velocity. (Derived from E=mc2 and E = hc/) = h_ mv Using the mass of an electron moving at the speed of light, he calculated the same energy level values as Bohr did for the Hydrogen atom. Louis de Broglie De Broglie realized that the electrons exhibit wavelike properties in their quantized orbits. (draw pictures ) He said that if an electron has wavelike motion and is restricted to circular orbits of a fixed radius, the electron is allowed only certain possible wavelengths, frequencies and energies. Experiments did show that electrons in atoms do exhibit wave behavior with specific frequencies. Standing wave animation The Heisenberg Uncertainty Principle Heisenberg concluded that it is impossible to make any measurement on an object without disturbing it – at least a little. Electrons are detected by photons and because a photon and an electron have the same energy, any attempt to locate an electron with a photon will knock the electron off course. Therefore: It is impossible to know both the position and the velocity of an electron at the same time. So we can only talk about the probability of finding and electron in certain area (remember the fuzzy cloud!) Erwin Schrodinger Used the idea that electrons behave like waves to write and solve a mathematical equation to describe the location and energy of an electron in the hydrogen atom. The modern description of the electron cloud in atoms comes from the solutions to the Schrodinger equation. This equation showed that the energy of electrons are restricted to certain values. Erwin Schrodinger However, the equation does not define the exact path the electron takes around the nucleus. It only estimates the probability of finding an electron in a certain position, unlike Bohr’s circular orbits. Electrons exist in regions called Orbitals Orbital – 3 dimensional cloud shape around the nucleus that indicates the probable location of an electron Atomic Orbitals The solutions to the Schrodinger equation are called Quantum Numbers, and they are used to describe the properties, such as the energy level and shape (s, p, d or f), and Orientation of the atomic orbitals. “s” - orbital • Each orbital’s shape is determined by it’s the electron’s energy, which is related to its wavelength!!!!! “p” orbitals An Electron in a p orbital has a different wavelength and therefore a different energy. This gives it a different shape!. “d” orbitals f – orbital = electron has a high energy, short wavelength! How many electrons can go into each orbital? Only 2 electrons are allowed in each orbital Each with an opposite spin. (this is to reduce repulsion between 2 negatively charged particles!) Quantum # and Atomic Structure Main Energy Level (n) Sublevels (n) *Orbital shapes # of orbitals per sublevel 1 s 2 s p 1 3 3 s p d 1 3 5 4 s p d f 1 3 5 7 1 # orbitals per main energy level (n2) # of electrons per sublevel Total # of electrons per main energy level (2n2) 1 2 2 4 s= 2 p= 6 8 9 s= 2 p=6 d = 10 18 16 s=2 p=6 d = 10 f = 14 32