* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download by Maillard Reaction
Chemical equilibrium wikipedia , lookup
Marcus theory wikipedia , lookup
Chemical thermodynamics wikipedia , lookup
Deoxyribozyme wikipedia , lookup
Woodward–Hoffmann rules wikipedia , lookup
Ene reaction wikipedia , lookup
Rate equation wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Supramolecular catalysis wikipedia , lookup
Transition state theory wikipedia , lookup
Industrial catalysts wikipedia , lookup
Photoredox catalysis wikipedia , lookup
George S. Hammond wikipedia , lookup
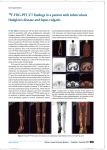
Synthesis of 18F-FDGly (18F-Fluorodeoxyglycosylamines) by Maillard Reaction (The Bread Browning Reaction) Aparna Baranwal Mentor: Jogeshwar Mukherjee 18F-Fluorodeoxyglucose (18F-FDG) is a radiopharmaceutical that is a common medical imaging agent used in positron emission tomography (PET). As a glucose analog, 18F-FDG can undergo the Maillard reaction with biologically significant amines. The Maillard reaction results in Amadori products that can be used for drug delivery and have a role in central nervous system. 18F-fluorodeoxyglycosylamines (18F-FDGly) were synthesized using the Maillard reaction of 18F-FDG with biological amines, under different reaction conditions. Respective amines N-allyl-2-aminomethylpyrroactionlidine (NAP) and 2-(4′-aminophenyl)-6hydroxybenzothiazole (PIB precursor) were mixed with FDG to provide fluorodeoxyglycosylamines, FDGNAP and FDGBTA. Radioactive 18F-FDGNAP and 18F-FDGBTA were synthesized using 18F-FDG with NAP and PIB precursor, respectively. Both FDGNAP and FDGBTA were isolated as stable products. Binding of FDGBTA and 18F-FDGBTA was then compared in postmortem human brain sections of Alzheimer’s disease (AD) patients and control subjects using autoradiography. Kinetics of the reaction 18FFDGNAP indicated increased product at 4 h (63% radiochemical yield). In addition, 18F-FDGBTA was prepared with 57% yield. Preliminary studies of FDGBTA showed displacement of 3H-PIB (reduced by 80%), and 18F-FDGBTA indicated selective binding to Aβ-amyloid plaques present in postmortem AD human brain, with a gray matter ratio of 3 between the AD patients and control subjects. We have demonstrated that 18F-FDG couples with amines under mild conditions to form 18F-FDGly in a manner similar to click chemistry. Although these amine derivatives are stable in vitro, stability and selective binding is under investigation.









![drug master file: [18f]fdg](http://s1.studyres.com/store/data/009298981_1-0014eb8c133d9a45f793a61c965c5722-150x150.png)