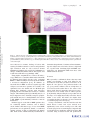
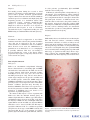
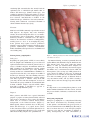
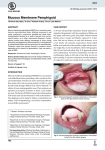
* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Pemphigoid diseases: Pathogenesis, diagnosis, and treatment
Adoptive cell transfer wikipedia , lookup
Hospital-acquired infection wikipedia , lookup
Anti-nuclear antibody wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Germ theory of disease wikipedia , lookup
Behçet's disease wikipedia , lookup
Signs and symptoms of Graves' disease wikipedia , lookup
Systemic scleroderma wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Globalization and disease wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Molecular mimicry wikipedia , lookup
IgA nephropathy wikipedia , lookup
Management of multiple sclerosis wikipedia , lookup
Pathophysiology of multiple sclerosis wikipedia , lookup
Multiple sclerosis signs and symptoms wikipedia , lookup
Immunosuppressive drug wikipedia , lookup
Neuromyelitis optica wikipedia , lookup
Autoimmunity wikipedia , lookup
Autoimmunity, February 2012; 45(1): 55–70 q Informa UK, Ltd. ISSN 0891-6934 print/1607-842X online DOI: 10.3109/08916934.2011.606447 Pemphigoid diseases: Pathogenesis, diagnosis, and treatment MICHAEL KASPERKIEWICZ1, DETLEF ZILLIKENS1, & ENNO SCHMIDT1,2 1 Department of Dermatology, University of Lübeck, Lübeck, Germany, and 2Comprehensive Center for Inflammation Medicine, University of Lübeck, Lübeck, Germany Autoimmunity Downloaded from informahealthcare.com by Goteborgs University on 11/20/12 For personal use only. (Submitted 25 June 2011; accepted 12 July 2011) Abstract Pemphigoid diseases (including bullous pemphigoid, mucous membrane pemphigoid, pemphigoid gestationis, linear IgA dermatosis, lichen planus pemphigoides, and anti-p200 pemphigoid) are a subgroup of autoimmune bullous skin diseases characterized by an autoantibody response toward structural components of the hemidesmosome resulting in subepidermal blistering. By the use of different in vitro systems and experimental animal models, the pathogenic relevance of these autoantibodies has been demonstrated. Recent advances in the understanding of autoantibody responses have led to novel diagnostic tools and a more differentiated therapeutic approach for these disorders. This review covers the most recent understanding of the pathophysiology, diagnosis, and treatment of this group of autoimmune diseases. Keywords: Pemphigoid, skin, antibody, antigen Introduction The pemphigoid group of diseases is characterized by subepidermal blisters due to autoantibody-induced disruption of the components of the dermal – epidermal anchoring complex. Pemphigoid diseases include bullous pemphigoid (BP), mucous membrane pemphigoid (MMP), pemphigoid gestationis (PG), linear IgA dermatosis (LAD), lichen planus pemphigoides (LPP), and anti-p200 pemphigoid. In particular, disorders with anti-BP180 (type XVII collagen) reactivity, including BP, PG, LAD, LPP, and a subgroup of MMP may form a continuous spectrum of subepidermal autoimmune blistering dermatoses. BP is not only the most common disorder within the pemphigoid group, but also represents the most frequent autoimmune blistering disease in general [1,2]. The incidence of BP has been estimated between 4.5 and 14 new cases/million/year in central Europe [1 –5]. A higher incidence of 42.8/million/year has recently been reported in Great Britain based on a data registry established on the general practitioner level [6]. Interestingly, in Germany and Great Britain, it has been shown that the incidence of BP has considerably increased within the last 10 years (2-fold and 4.8-fold, respectively) [1,6]. This development may be due to the increasing age of the general population and/or an increased awareness leading to further diagnostic steps. MMP and PG have been identified as the second most frequent pemphigoid diseases in central Europe with an incidence of two new patients/million/year [1]. For the correct diagnosis of pemphigoid diseases, the detection of tissue-bound and circulating autoantibodies is pivotal [7]. Although direct immunofluorescence (IF) microscopy differentiates pemphigus from pemphigoid disorders, serological analyses are necessary to separate pemphigoid diseases from each other and from epidermolysis bullosa acquisita (EBA). In fact, while direct IF microscopy is still the gold standard for the diagnosis of pemphigoid diseases, in the great majority of patients, diagnosis can be made serologically today [7]. By indirect IF microscopy on 1 M NaCl-split human skin, anti-laminin 332 MMP and anti-p200 pemphigoid can be distinguished from BP, LAD, PG, and anti-BP180 MMP, but final diagnosis can only be made by more sophisticated methods, i.e. use of cell-derived or recombinant forms of the target antigens (Figure 1). In recent years, various novel detection systems for serum antibodies have been Correspondence: M. Kasperkiewicz, Department of Dermatology,University of Lübeck, Ratzeburger Allee 160, 23538 Lübeck, Germany. Tel: 0049-451-500-5844. Fax: 0049-451-500-5162. E-mail: [email protected] 56 M. Kasperkiewicz et al. (a) Disease Main target antigens Bullous pemphigoid BP180, BP230 Mucous membrane pemphigoid BP180, BP230, α6β4 integrin Pemphigoid gestationis BP180, BP230 Linear IgA dermatosis BP180, BP230 Lichen planus pemphigoides BP180, BP230 Autoimmunity Downloaded from informahealthcare.com by Goteborgs University on 11/20/12 For personal use only. (b) Disease Main target antigens Mucous membrane pemphigoid Laminin 332 Anti-p200 pemphigoid Laminin γ1 Figure 1. Indirect IF microscopy on 1 M NaCl split human skin and main target autoantigens of pemphigoid diseases. Differentiation of the diseases and target molecules is based on the binding pattern with either (a) epidermal or (b) dermal binding of circulating autoantibodies on the artificial split. developed, some of which are also commercially available. These now allow for a more accurate diagnosis, which is becoming increasingly important to guide treatment decisions, while our knowledge about the therapeutic arsenal of these disorders has also expanded. In this review, we focus on recent progress in our understanding of the pathogenesis, diagnosis, and treatment of the different pemphigoid diseases. Bullous pemphigoid Pathogenesis Two hemidesmosomal proteins, the 230 kDa protein (BP230 or BPAG1), and 180 kDa antigen (BP180, BPAG2, or type XVII collagen) have been identified as the major antigenic targets of BP autoantibodies [8–10]. BP230 is an intracellular plakin protein member showing homology with plectin and desmoplakins I and II and promotes the association of hemidesmosomes with keratin intermediate filaments [11–13]. In contrast, BP180 is a transmembrane protein with a type II orientation that spans the lamina lucida and projects into the lamina densa of the epidermal basal membrane zone (BMZ). The extracellular region consists of 15 collagen domains separated from one another by noncollagen sequences [14,15]. The noncollagenous 16A domain (NC16A), located at the membrane-proximal region of BP180, is considered to harbor major pathogenically relevant epitopes in BP and is recognized by autoantibodies in 80 – 90% of BP patients [16 –19]. The importance of anti-BP180 NC16A reactivity is further highlighted by the observation that serum levels of BP180 NC16A-specific antibodies correlate with the disease activity in BP patients [20]. In addition, it was reported that autoantibodies preferentially recognize the phosphorylated BP180 ectodomain [21,22]. Recent studies have identified the presence of memory B cells specific for the NC16A domain, which can be induced in vitro to synthesize autoantibodies [23]. These cells belong to the shortlived plasma blast population [24]. The BP180 NC16A-specific autoantibodies are not only of the IgG isotype (mainly IgG1 and IgG4 subclasses), but also of the IgE class [25 –27]. In fact, patients with BP frequently have IgE autoantibodies binding to the NC16A domain of BP180 [28]. The presence of IgE autoantibodies has recently been correlated with a severe form of BP, and BP patients, positive for IgE anti-BP180 antibodies, required longer duration for remission, higher dosage of prednisolone, and more intensive therapies for remission [29]. Interestingly, one study showed that a recombinant NC16A fusion protein degranulated basophils opsonized with IgE from patients with BP [30], further supporting the pathophysiological role of IgE antibodies in BP. In addition, other antigenic sites exist on both the extracellular and intracellular Autoimmunity Downloaded from informahealthcare.com by Goteborgs University on 11/20/12 For personal use only. Update on pemphigoid domain of BP180, which are recognized by IgE and IgG autoantibodies in BP patients [16 –18,31 – 34]. BP patients also exhibit significant reactivity with BP230, which is thought not to be involved in the initiation of the inflammatory response in BP due to its intracellular localization [8,35 –40]. Currently, it is unclear whether anti-BP230 autoantibodies in BP patients directly contribute to blister formation or whether they are just a result of keratinocyte injury and determinant spreading of the autoimmune response. Very recently, two retrospective studies with a large series of 138 and 190 patients with active BP, respectively, have determined the outcome of BP230 autoantibody detection using commercial enzymelinked immunosorbent assays (ELISAs) [39,40]. In the first-mentioned study, 59% of patients had a positive BP230 ELISA result and 86% had a positive BP180 ELISA result, while only 5% had anti-BP230 autoantibodies without anti-BP180 autoantibodies [39]. In the second study, anti-BP230 and antiBP180 antibodies were detected in 61 and 79% of BP patients serum samples, respectively, while 8% had anti-BP230 autoantibodies without anti-BP180 autoantibodies [40]. Although the study by Charneux et al. found no relationship between a positive BP230 ELISA result and disease extent or presence of mucosal involvement [39], with regard to this specific finding, the study by Roussel et al. [40] showed the opposite finding. Two other studies suggested that anti-BP230 antibodies may be preferentially associated with localized types of BP [37,38]. In contrast to the humoral immune response, the cellular immune response has been less widely studied in human BP. Autoreactive T and B cells are almost constantly found in BP patients. These cells react with the same regions of BP180 and BP230 that are recognized by IgG autoantibodies. The differential epitope recognition of BP180 seems to be associated with distinct clinical severity: T- and B-cell reactivity against the NH2-terminal portion of the BP180 ectodomain is associated with severe BP, while the central portion is more frequently recognized in patients with limited disease. In contrast, combined T- and B-cell response against the COOH- and NH2-terminal globular domains of BP230 was found in less than 50% [41]. The response to the BP180 ectodomain is restricted by certain HLA class II alleles, such as the DQb1*0301 allele [42,43]. Autoreactive T cells in BP patients produce a Th1/Th2 mixed cytokine profile. We and others have detected elevated levels of IL-1b, IL-2, IL-4, IL-5, IL-6, IL-8, IL-10, IL-15, IL-16, eotaxin, MCP-4, tumor necrosis factor a (TNFa), and CCL18 in the sera and/or blister fluids of BP patients [44 –55]. Serum levels of TNFa, IL-6, IL-8, IL-15, and CCL18 correlated with patients’ disease activity [47– 49,54], pointing to a pathological relevance of these mediators. The observation that Th2-type cytokines 57 are important in human BP is supported by the increased frequency of cutaneous lymphocyte-associated antigen-positive IL-4- and IL-13-producing cells in the peripheral blood [56]. Furthermore, analysis of cell subsets with immunoregulatory functions in BP patients has shown that while the number of circulating CD4 þ CD25 þ FOXP3 þ regulatory T cells, natural killer T cells, and natural killer cells are normal, gd T cells are reduced in BP patients [57,58]. Our knowledge about the functionally relevant pathogenic mechanisms in BP is based on in vitro studies using cultured human keratinocytes, ex vivo studies using cryosections of human skin as well as various mouse models [59,60]. When cultured human keratinocytes were treated with anti-BP180 IgG, doseand time-dependent increases of both mRNA and protein levels of IL-6 and IL-8 were observed, pointing to a signal-transducing event via BP180 [61]. In the same model, decreased expression of BP180 and weakening of keratinocyte attachment in response to anti-BP180 IgG were seen [62]. Using cryosections of human skin, BP patients’ sera were shown to generate dermal –epidermal separation when co-incubated with leukocytes and complement from healthy volunteers [63]. Characterizing the specificity of pathogenically relevant autoantibodies, we were later able to demonstrate that IgG antibodies to human BP180, purified from sera of BP patients and from rabbits immunized against recombinant human BP180, induce dermal– epidermal separation in this model. This effect was shown to be mediated by binding of autoantibodies to the NC16A domain of human BP180 and to be dependent on the Fc portion of autoantibodies [64]. Passive transfer of rabbit IgG, raised against the murine homolog of the human BP180 NC16A domain, into neonatal wild-type mice produced clinical, histologic, and immunopathologic alterations like those seen in patients with BP [65]. In this model, blister formation is dependent on the activation of complement, degranulation of mast cells, and recruitment of neutrophils [65 –68]. Blister fluid and lesional/perilesional biopsies from BP patients have been shown to contain high levels of proteolytic enzymes, including neutrophil elastase (NE), cathepsin G, collagenase, plasminogen activators, plasmin, matrix metalloproteinase-2 (MMP-2, gelatinase A), MMP-9 (gelatinase B), and MMP-13 [69 – 77]. These enzymes, particularly the neutrophiland eosinophil-derived MMP-9 and NE, are secreted into the extracellular space upon cell activation and are thought to proteolytically degrade various extracellular matrix proteins, including the extracellular domain of BP180 [76,78,79]. Mice genetically deficient in NE or MMP-9 have been shown to be resistant to experimental BP [80,81]. An interaction between MMP-9, NE, and the plasminogen/plasmin system was demonstrated to Autoimmunity Downloaded from informahealthcare.com by Goteborgs University on 11/20/12 For personal use only. 58 M. Kasperkiewicz et al. occur during proteolytic events in experimental BP: in the early stages of blistering, MMP-9 is mainly activated by plasmin, which is formed from plasminogen by tissue plasminogen activator (tPA) and/or urokinase plasminogen activator (uPA) [82]. Furthermore, an elevated expression and release of tPA from normal human keratinocytes upon stimulation with antibodies to human BP180 has been reported [83]. In addition to plasmin, the mast cell-specific serine protease MCP-4 (chymase) is also able to activate MMP-9 [84]. Activated MMP-9 is believed to proteolytically inactivate a1-proteinase inhibitor, the physiological inhibitor of NE, which allows unrestrained activity of NE [85]. These findings demonstrate that loss of cell-matrix adhesion within the dermal-epidermal junction (DEJ) is directly mediated by proteinases released by inflammatory cells. In a first attempt to explore the functional relevance of human anti-BP180 antibodies reacting with the human target antigen, human skin was transplanted onto Severe Combined Immunodeficiency (SCID) mice. The injection of BP IgG into the transplant did, however, not result in blister formation within the observation period of 48 h [86]. Recently, additional “humanized” mouse models have been established. Olasz et al. generated a novel transgenic mouse expressing human BP180 in murine epidermal BMZ by the use of a keratin 14 promoter construct. They showed that wild-type or MHC I2/2 , but not MHC II2/2 , mice grafted with transgenic skin elicited IgG that bound human epidermal BMZ and BP180. Transgenic grafts on wild-type mice developed neutrophil-rich leukocyte infiltrates and subepidermal blisters [87]. Nishie et al. [88] used the same BP180 transgenic mice to rescue the blistering phenotype of BP1802/2 mice by backcrossing experiments. Clinical, histological, and immunopathologic features of BP were achieved by passive transfer of IgG from patients or IgG1 monoclonal antibodies against humanized BP180 NC16A into these BP180-humanized mice [88,89]. To show that pathogenic antiBP180 autoantibodies act in combination with key innate immune system players also in the humanized BP mouse model, Liu et al. generated a humanized mouse strain, in which murine BP180 NC14A was replaced by the homologous human BP180 NC16A epitope [90]. These BP180 humanized mice pretreated with mast cell activation blocker or depleted of complement or neutrophils become resistant to BP after passive transfer of human BP autoantibodies. More recently, an active mouse model for BP has been developed by transferring splenocytes from wild-type mice that had been immunized by grafting of human BP180-transgenic mouse skin onto Rag-22/2 /BP180humanized mice. The recipient mice produced anti-human BP180 IgG antibodies in vivo and developed blisters and erosions corresponding to clinical, histological, and immunopathological features of BP [91]. In two other mouse models, the pathogenic effect of IgE anti-BP180 antibodies has been elucidated, demonstrating that an IgE hybridoma to LABD97 antigen or purified IgE from BP patients was able to induce BP-characteristic inflammatory skin changes and partly subepidermal blistering in human skin grafts on nude mice [92,93]. Diagnosis Clinically, BP is characterized by tense blisters predominantly on flexural aspects of the limbs and abdomen and associated with severe itching (Figure 2a). BP typically affects the elderly with an average age at diagnosis between 75 and 81 years [1,2,94,95]. Before blisters arise, BP is typically preceded by a prodromal, non-bullous stage, in which excoriated, eczematous, papular, and/or urticarial lesions are found that may persist for several weeks or even months. Light microscopy of lesional skin from patients with BP typically demonstrates a subepidermal blister with an eosinophil- and/or neutrophil-rich leukocytic infiltrate within the papillary dermis and along the epidermal BMZ (Figure 2b). By direct IF microscopy of perilesional skin linear deposits of IgG and/or C3 can be found at the epidermal BMZ (Figure 2c). In approximately 80% of the cases, patient IgG autoantibodies react with the epidermal side of 1 M NaCl split human skin by indirect IF microscopy (Figure 2d) [96]. Based on the finding that NC16A is the immunodominant region of BP180 [17,18], two highly specific and sensitive BP180 NC16A-specific ELISAs for detection of circulating autoantibodies in BP have been commercialized [19,97]. When these assays are combined with other detection systems, including those using BP180 fragments outside the NC16A domain and BP230, the sensitivity of these tests can be increased to even 100% [38,41,98,99]. In contrast to serum levels of antibodies to BP230, BP180 NC16Aspecific autoantibodies correlate closely with disease activity and may thus be not only of diagnostic use, but also allow the monitoring of circulating autoantibody levels during the course of the disease, helping to guide treatment decisions [19,20,36]. BP must be differentiated from pemphigus and other subepidermal blistering autoimmune dermatoses, including EBA and dermatitis herpetiformis. In contrast to BP, blister formation in pemphigus results from intraepithelial cell separation and shows an intercellular pattern of IgG between keratinocytes. Differentiation of BP especially from EBA, anti-p200 pemphigoid, and MMP, however, presents a more difficult task. The mechanobullous, non-inflammatory form of EBA is characterized by the appearance of skin fragility and tense blisters at anatomic Autoimmunity Downloaded from informahealthcare.com by Goteborgs University on 11/20/12 For personal use only. Update on pemphigoid 59 Figure 2. Clinical, histologic, and immunopathologic findings in BP as representative pemphigoid disease. (a) Tense blisters and erosion on erythematous base on the leg. (b) Histophathology of lesional skin demonstrating a subepidermal blister and a mixed dermal inflammatory infiltrate containing numerous granulocytes. (c) Direct IF microscopy of perilesional skin showing continuous linear deposits of IgG at the epidermal BMZ. (d) Indirect IF microscopy on 1 M NaCl split human skin revealing reactivity of circulating IgG against the epidermal side of the artificial split. areas subjected to trauma, healing of lesions with scarring and milia formation as well as subepidermal split formation with an only scattered dermal inflammatory infiltrate. In contrast, the inflammatory subtype of EBA may mimic clinical, histological, and routine direct IF microscopy findings of BP. Using higher magnifications of direct IF microscopy, EBA can be sometimes differentiated from BP by distinctly shaped IgG deposits at the epidermal BMZ, i.e. EBA revealing an u-serrated pattern and BP a n-serrated configuration [100]. In addition, as autoantibodies in EBA are directed against type VII collagen, sera from these patients do not bind to the epidermal, but to the dermal side of 1 M NaCl split human skin. Similarly, patients with anti-p200 pemphigoid show autoantibody reactivity to a dermal antigen. This immunopathological finding may be of major importance since clinically, anti-p200 pemphigoid and BP may not be distinguishable. In contrast to BP, MMP is predominantly confined to mucous membranes. If blisters appear on the skin of MMP patients, they are commonly smaller, transient, and of limited extent. Indirect IF microscopy of sera from MMP patients may reveal epidermal, dermal, or combined staining patterns of IgG on 1 M NaCl split human skin. Some forms of LAD clinically resemble BP or dermatitis herpetiformis. Continuous linear immunodeposits of IgA, most often in the absence of IgG and C3, together with circulating IgA anti-BMZ autoantibodies, can distinguish LAD from these other immunobullous diseases. Treatment BP is generally a self-limited disease that may remit within a few months or some years. However, the mortality rate in the first year has been shown to be as high as 11 – 40% [95,101 – 103], and was related to old age, poor general condition, and use of high doses of oral corticosteroids rather than to the extent of the disease itself [94,102]. Systemic and topical corticosteroids have been widely used in clinical practice [104]. Initially, daily morning doses of prednisolone in the range of 0.5 mg/kg/day usually control the disease within a few weeks. An initial dose higher than 0.75 mg/kg/day has been shown to be not beneficial in a controlled prospective trial [105]. A large, randomized controlled trial showed that initial disease control and 1-year survival were significantly better when treating severe BP with clobetasol propionate cream 40 mg/day compared with oral prednisolone 1 mg/kg/day, while in moderate BP, outcomes using clobetasol cream and prednisolone Autoimmunity Downloaded from informahealthcare.com by Goteborgs University on 11/20/12 For personal use only. 60 M. Kasperkiewicz et al. 0.5 mg/kg/day were comparable [106]. Furthermore, a mild regimen with clobetasol propionate cream 10 – 30 g/day tapering over 4 months has been shown to be not inferior to the high-dose topical regimen with clobetasol propionate 40 g/day [107]. Considering other randomized controlled trials in BP, no differences in disease control were seen for azathioprine plus prednisone compared with prednisone alone [108], for prednisolone plus azathioprine compared with prednisolone plus plasma exchange [108], for prednisolone plus mycophenolate mofetil or plus azathioprine [109], and for tetracycline plus nicotinamide compared with prednisolone [110]. Reports have also described successful treatment of BP patients with dapsone [111,112], methotrexate [113], high-dose intravenous immunoglobulins (IVIG) [114], rituximab [115 – 117], omalizumab [118], and immunoadsorption [119]. Mucous membrane pemphigoid Pathogenesis BP180 is the target antigen in about 70% of MMP patients [120]. However, unlike in BP, only about half of the patients’ sera contain antibodies to BP180 NC16A, and C-terminal epitopes of BP180 are preferentially targeted [120 – 122]. In addition, patients with MMP may exhibit IgG and/or IgA autoantibodies of other specificity that recognize BP230 [123,124], the 97/120 kDa LAD antigen, laminin 332 (laminin 5), laminin 331 (laminin 6) [125,126], type VII collagen [127], or the a6 and b4 integrin subunit [128]. Considering the latter target antigens, it has been shown that sera from patients with generalized MMP and ocular MMP recognize the b4 integrin subunit, while sera from patients with oral MMP recognize the a6 integrin subunit [129]. The pathogenicity of anti-laminin 332 antibodies has been documented in vivo. Passive transfer of antilaminin 332 IgG to neonatal or adult mice induced subepidermal blisters of skin and mucous membranes that mimicked clinical, histological, and immunopathologic features seen in MMP patients [130]. The same findings were seen in mice injected with Fab fragments directed against laminin 332 as well as in mice lacking complement, mast cells or T cells, suggesting that such antibodies may elicit epidermal detachment in vivo in a non-inflammatory and direct manner [130,131]. The pathogenicity of patients’ autoantibodies was further confirmed in an experimental human skin graft model, in which human anti-laminin 332 autoantibodies induced subepidermal blisters [132]. Additionally, accumulating evidence indicates that fibrogenic cytokines, matrix metalloproteinases, and collagen type I secreted in high amounts by pemphigoid fibroblasts may actively be involved in ocular MMP-associated scarring processes [133,134]. Diagnosis MMP is a chronic autoimmune blistering disease of mucous membranes and skin. An international consensus conference defined the predominant involvement of mucous membranes as the major clinical diagnosis criterion of MMP [135]. The oral mucosa is the most common site affected by MMP followed by ocular disease (Figure 3). It may also involve nasal, pharyngeal, laryngeal, esophageal, and anogenital mucous membranes. Progressive scarring potentially may lead to serious complications, such as blindness or asphyxiation. Histologically, MMP is characterized by subepidermal blisters with a chronic inflammatory infiltrate in the lamina propria that is composed of lymphocytes, histiocytes, scattered neutrophils, and eosinophils. Anti-BP180 and anti-laminin 332 MMP cannot be differentiated from each other by histology [136]. Direct IF microscopy shows linear IgG and C3 deposits at the BMZ. Indirect IF microscopy using 1 M NaCl split human skin distinguishes between antigens on the epithelial side of the split (BP180, BP230, and a6b4 integrin) and those of the dermal side (laminin 332). However, as only about one-half of sera are reactive in indirect IF microscopy, ELISA and Western blotting using relevant target antigens are important additional diagnostic methods in MMP. Although only half of the MMP patients develop antibodies to the NC16A domain of BP180, the other patients with BP180 reactivity react with LAD-1 or the C-terminal portion of BP180 by Western blotting [130]. Importantly, testing for IgA anti-BP180 antibodies in addition to the IgG isotype will further increase the detection rate [130,137]. Assaying for anti-laminin 332 reactivity is of particular importance as 25% of patients with laminin 332-specific antibodies develop a malignancy. In these Figure 3. Clinical presentation of MMP. Ocular involvement with symblepharon formation. Autoimmunity Downloaded from informahealthcare.com by Goteborgs University on 11/20/12 For personal use only. Update on pemphigoid patients, a tumor search is indicated [138,139]. Immunoprecipitation with human keratinocytes has been reported to be the most sensitive technique for the detection of serum autoantibodies against laminin 332, whereas immunoblotting with extracellular matrix of cultured HaCaT cells was found to be the most practical alternative [140]. The term cicatricial pemphigoid, previously applied for patients with MMP, is currently only used for the rare clinical variant in which mucous membranes are not predominantly affected and skin lesions heal with scarring [135]. Brunsting –Perry pemphigoid is a rare variant of cicatricial pemphigoid first described in 1957 [141]. It is characterized clinically by blisters, hemorrhagic crusts and atrophic scars confined to the head and neck without mucosal involvement. It probably represents a heterogeneous disorder with several target antigens. Most patients with this type of pemphigoid disease have been reported to have autoantibodies against type VII collagen, therefore, representing a localized form of EBA [142]. Although the clinical features of Brunsting – Perry pemphigoid are completely different from those of MMP, it has recently been reported that they may share the same target antigens, e.g. the C-terminal domain of BP180 and laminin 332 [143,144]. Treatment Treatment of MMP is largely tempered by its severity and sites of involvement. A consensus conference on this disease differentiated high-risk and low-risk patients. Although in low-risk patients, lesions are limited to the oral cavity and the skin and are more amenable to treatment, patients with conjunctival involvement usually require aggressive management to avoid blindness. Mild lesions of the oral mucosa and skin can sometimes be effectively treated with topical glucocorticoids or topical calcineurin inhibitors combined with good oral hygiene. For more severe lesions in low-risk patients, initial treatment may include dapsone (1.0 – 1.5 mg/kg/day) and prednisolone (0.5 – 1.0 mg/kg/day). An alternative for these patients may also be tetracyclines combined with nicotinamide. Prednisolone (1.0 –1.5 mg/kg/day) in combination with oral cyclophosphamide (1.0 –2.0 mg/kg/day) may be tried in patients at high risk (ocular, nasopharyngeal, genital, and esophageal involvement) [145,146]. Alternatively, i.v. dexamethasone pulses (100 mg on 3 consecutive days) combined with i.v. cyclophosphamide pulses (500 –1000 mg) in 2- to 4-week intervals can be given. In patients who do not tolerate cyclophosphamide, other immunosuppressants such as azathiorpine, mycophenolate mofetil, and methotrexate can be applied [145,146]. Good therapeutic efficacy of IVIG has been reported in patients with MMP. IVIG therapy, usually 61 given at 2 g/kg/cycle initially every 4 weeks, avoided progression of the disease and was more effective than conventional immunosuppressive treatment [104]. In one study, IVIG given as monotherapy halted progression of scarring and visual deterioration during the study period, and serological and clinical remission was sustained for several months after cessation of IVIG infusions [147]. Recent case reports also described the beneficial use of rituximab and TNF-a inhibitors for severe and treatment-resistant cases of MMP [105,107,148]. Pemphigoid gestationis Pathogenesis PG, formerly referred to as herpes gestationis, is another disease in which BP180 has been identified as the major target [149]. Occasionally, PG is associated with a trophoblastic tumor, hydatidiform mole or choriocarcinoma. The disease may appear for the first time during any pregnancy, but then rarely skips subsequent gestations [149]. The immunopathologic hallmark of PG is linear deposits of C3 and, to a lesser extent, of IgG along the DEJ in perilesional skin biopsies. Circulating-complement fixing autoantibodies “HG factor” can be detected by indirect IF on intact or NaCl split skin. The autoantibodies react with BP180 and, less frequently, with BP230 [16,150]. IgG autoantibodies in most PG sera target the NC16A domain of BP180 [16,32,151 – 153]. However, antigenic sites outside NC16A have also been identified both extra- and intracellularly [153]. Epitope mapping studies identified two well-defined antigenic sites within a 22 amino acid segment of BP180 (NC16A2 and NC16A2.5) as major antigenic targets for PG sera [152]. In addition, using overlapping synthetic peptides, PG autoantibodies were shown to bind to two defined epitopes within the NC16A domain (aa 500 – 514 and aa 511 – 523). Importantly, preadsorption using an affinity matrix containing these epitopes completely abolished dermal– epidermal separation ex vivo induced by PG autoantibodies [154]. In contrast to findings in BP [155], reactivity to NC16A is dominated by IgG1 and IgG3 antibodies in PG patients [152]. As IgG1 and IgG3 are the IgG subclasses with the strongest complement fixing properties, these observations may well explain complement deposition at the DEJ, which is the most consistent immunopathological feature in PG. In PG patients tested for T-cell autoreactivity, BP180specific T cells exclusively reacted with the NC16A2 domain. Proliferative responses of these cells were restricted to HLA-DR and cells expressed a CD4 þ Th1-type memory phenotype [156]. These results underline other findings that PG is strongly associated with HLA-DR3 and -DR4 haplotypes [149]. 62 M. Kasperkiewicz et al. Diagnosis Autoimmunity Downloaded from informahealthcare.com by Goteborgs University on 11/20/12 For personal use only. PG usually presents during the second or third trimester of pregnancy or in the immediate postpartum period with a pruritic urticarial, papulovesicular eruption. Skin lesions often start around the umbilicus and then spread over abdomen and thighs [149]. PG frequently presents as a nonbullous disease with eczematous lesions, erythema multiforme-like changes or erythematous papules and plaques. Diagnosis is based on detection of C3 deposits in perilesional skin biopsies as well as circulating autoantibodies by the complement binding test and ELISA using recombinant BP180 NC16A [19,157]. Treatment Treatment is aimed at suppression of new blister formation and relief of the intense pruritus. In milder cases, this can be achieved by the use of topical corticosteroids in combination with oral antihistamines. In more severe cases, the administration of prednisolone at an initial dose of 0.3 – 0.5 mg/kg/day is generally sufficient. This dose can usually be decreased during the course of the disease. Flares postpartum may require a temporary increase in the corticosteroid dose [149]. in older patients, predominantly IgG anti-BMZ antibodies were found [173]. LAD may be either idiopathic or drug-induced (e.g. vancomycin). The mechanism for blister formation is not fully understood, but is likely to involve IgAand complement-mediated neutrophil chemotaxis. The pathogenic relevance of LAD-associated autoantibodies has been shown by the passive transfer of IgA murine monoclonal antibodies against LAD autoantigen to SCID mice bearing human skin grafts. In some of these challenged mice, the transferred autoantibodies produced neutrophil-rich infiltrates and subepidermal vesicles [174]. Diagnosis LAD mainly shows vesicobullous lesions affecting the skin and mucosal surfaces, sometimes forming characteristic collarettes of vesicles or blisters as new lesions arise in the periphery of old lesions (Figure 4). On histopathology, the bullae are subepidermal, with collections of neutrophils along the epidermal BMZ and occasionally in the dermal papillary tips. Direct IF microscopy of perilesional skin shows linear deposition of IgA at the epidermal BMZ, sometimes in combination with linear IgG deposits. Patients have Linear IgA dermatosis Pathogenesis LAD is an autoimmune subepidermal blistering disease characterized by circulating IgA anti-BMZ autoantibodies. Autoantibodies in LAD were shown to target antigens with various molecular weights, including 97-, 120-, 180-, 200-, 230-, 280-, 285- and 290-kDa proteins [158 –160]. The two most characteristic target antigens are the protein of 97 kDa and the 120 kDa protein, termed the LABD antigen 1 (LABD97) and LAD-1, respectively [159,161]. These proteins present a cleaved portion of the extracellular domain of BP180 [160,162 – 165]. Recently, the sheddases ADAM 9 and 10 have been reported to be involved in generation of LAD-1 from BP180, while formation of LABD97 has been shown to be dependent on plasmin [166,167]. In contrast to BP, only 20% of sera of patients with LAD react with the NC16A domain [168]. In addition, IgA antibodies to BP230, type VII collagen and laminin 332 have been reported [169– 171]. Since the majority of LAD sera also contain IgG antibodies against BP180 and in most BP sera, IgA anti-BP180 antibodies can be detected, LAD and BP may be regarded as different ends of a continuous spectrum [172]. In fact, the isotype of anti-BMZ reactivity was associated with the age of the patients: in younger patients, IgA autoantibodies predominated, whereas Figure 4. Clinical presentation of LAD. Erythema multiforme-like lesions with partly clustered annular tense blisters on proximal lower extremity. Update on pemphigoid 63 circulating IgA autoantibodies that mostly bind the epidermal side of 1 M NaCl split human skin on indirect IF microscopy (lamina lucida type), but combined epidermal and dermal staining as well as dermal labeling alone (sublamina densa type) has also been observed. Autoantibodies to LAD-1 in the lamina lucida type of LAD are detected by immunoblotting with conditioned concentrated medium of cultured human keratinocytes [159]. Autoimmunity Downloaded from informahealthcare.com by Goteborgs University on 11/20/12 For personal use only. Treatment Skin lesions in LAD commonly respond when treated with dapsone. As dapsone may cause hemolytic anemia, decreased hemoglobin values or even methemoglobinemia, glucose-6-phosphate dehydrogenase deficiency should be excluded before the drug is initiated. An alternative treatment is Sulfapyridine, usually given at a dose of 15 – 60 mg/kg/day [175]. Some patients may require low-dose prednisone initially to suppress blister formation. In unresponsive patients, erythromycin, colchicine, flucloxacillin, IVIG, and immunoadsorption have been administered successfully [176]. Lichen planus pemphigoides Pathogenesis Regarding the pathogenesis of LPP, it is most likely that the lymphocytic inflammatory process directed against basal keratinocytes in lichen planus leads to a release of hidden antigenic determinants within the DEJ, resulting in an autoimmune response against hemidesmosomal structures [177 – 180]. Immunoblotting studies have identified BP180 and BP230 as target molecules but also a new antigen of 200 kDa of keratinocyte derivation. The latter finding reinforces the hypothesis that LPP might have a unique antigen and thus represent a distinct entity from BP [178,179]. In line with this assumption, it has been shown that the epitope within the C-terminal NC16A domain of BP180 antigen, targeted in LPP, differs from BP, localizing to NC16A4 as opposed to NC17A1 through NC16A3 [180]. Diagnosis Most patients with LPP have typical lichenified plaques or papules of lichen planus initially, followed after weeks to months by tense vesicles and blisters which arise, in contrast to bullous lichen planus, independent of the lichenoid lesions (Figure 5). Histologically, blisters resemble those in BP [177]. Direct IF microscopy of a papule shows Civatte bodies, but when a blister is evaluated, IgG and C3 are present as in BP. Indirect IF microsocopy on salt split skin shows binding of IgG to the epidermal side. Figure 5. Clinical presentation of LPP. Lichenified plaques and tense blisters on the foot. By immunoblotting, reactivity is primarily directed against BP180 with NC16A being the immunodominant domain [180]. Less often, antibodies against BP230 or other yet uncharacterized antigens are found [177– 179]. The following observations suggest that LPP is not a coincidental association of BP and lichen planus: (i) the average age of BP patients is 77 years, whereas in LPP it is 44 years; (ii) lichenoid papules are not seen in BP; (iii) autoantibodies in LPP react with C-terminal epitopes of NC16A, which is very uncommon in BP; and (iv) LPP is usually a milder disease and more therapy-responsive than BP. Therapy It is important to treat existing lichen planus to avoid further immunostimulation. In this context, acitretin has proven valuable. Otherwise, treatment is the same as in BP. Anti-p200 pemphigoid Anti-p200 pemphigoid is a subepidermal blistering skin disease characterized by circulating autoantibodies against a 200-kDa molecule (p200 protein) of the lower lamina lucida of the BMZ [181]. Recently, it has been shown that 90% of the anti-p200 pemphigoid sera react with the C-terminus of laminin g1 [182]. Subsequently, the term anti-laminin g1 64 M. Kasperkiewicz et al. artificial blister by indirect IF microscopy on 1 M NaCl split human skin [181]. Classically, autoantibodies in anti-p200 pemphigoid react with a 200-kDa protein in extracts of human dermis by Western blotting. Recently, laminin g1-specific antibodies could be detected by Western blotting or ELISA using the recombinant C-terminus of laminin g1 [182,183]. Autoimmunity Downloaded from informahealthcare.com by Goteborgs University on 11/20/12 For personal use only. Treatment Figure 6. Clinical presentation of anti-p200 pemphigoid. Tense blisters and erosions on the palms. pemphigoid was proposed for this disease. Because not all anti-p200 pemphigoid sera react with laminin g1 [182,183], we suggest not applying the two terms synonymously and only diagnosing an anti-laminin g1 pemphigoid when reactivity with laminin g1 has actually been identified. Pathogenesis Anti-p200 pemphigoid shows organ-specific pathogenicity, although laminin g1 is expressed not only at the DEJ but also in the BMZ of dermal blood vessels [184]. This has been explained by the assumption that laminin g1 in the epidermal BMZ has different posttranslational modifications, such as glycosylation, compared with laminin g1 expressed in blood vessels [185]. However, the pathogenicity of anti-p200/laminin g1 antibodies has hardly been studied yet, with the exception of a single patient whose IgG, in contrast to BP IgG, did not induce IL-8 secretion following incubation with human cultured keratinocytes [186]. Anti-p200 pemphigoid can be treated in a similar way as BP and typically shows a prompt response to topical class IV corticosteroids and dapsone (1.0 – 1.5 mg/kg/day). In more severe cases, prednisolone (0.5 mg/kg/day) may be added [187]. Recently, adjuvant immunoadsorption has been successfully applied in a severe case of anti-p200 pemphigoid with concomitant active gastric ulceration [189]. Conclusion Considerable progress has been made in the last years regarding our understanding of pemphigoid diseases. Experimental animal models of these diseases and advances in translational research provided essential insight into the pathophysiology of these disorders. Intense scientific research on the autoantibody response has led to new diagnostic techniques, allowing the serological diagnosis in the great majority of patients. Novel, more specific therapeutic avenues are, however, needed to further reduce the long-term adverse effects of the currently available immunosuppressants. Declaration of interest: This work was supported by grant EXC 306/1 Inflammation at Interfaces (Research Areas E and H) from the Deutsche Forschunggemeinschaft to E.S. and D.Z. The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper. Diagnosis Patients with anti-p200 pemphigoid typically develop tense blisters and urticarial eruptions, sometimes with itching, closely resembling those of BP (Figure 6). Usually, patients with anti-p200 pemphigoid can only be clinically differentiated from BP by their considerably younger age with an average of 61 years at diagnosis [187]. Histopathologically, skin biopsy specimens demonstrate subepidermal blistering and a linear infiltrate of neutrophils, and sometimes eosinophils, along the epidermal BMZ. With this method, anti-p200 pemphigoid cannot be differentiated from other pemphigoid disorders [188]. Direct IF microscopy of perilesional skin biopsies from patients with p200 pemphigoid demonstrate linear deposits of IgG and C3 along the epidermal BMZ. Typically, autoantibodies bind to the floor of the References [1] Bertram F, Bröcker EB, Zillikens D, Schmidt E. Prospective analysis of the incidence of autoimmune bullous disorders in Lower Franconia, Germany. J Dtsch Dermatol Ges 2009;7: 434–440. [2] Marazza G, Pham HC, Schärer L, Pedrazzetti PP, Hunziker T, Trüeb RM, Hohl D, Itin P, Lautenschlager S, Naldi L, Borradori L. Autoimmune bullous disease Swiss study group. Incidence of bullous pemphigoid and pemphigus in Switzerland: A 2-year prospective study. Br J Dermatol 2009; 161:861–868. [3] Cozzani E, Parodi A, Rebora A, Delmonte S, Barile M, Nigro A, Priano L, Troiano G, Patri PL, Gruppo Ligure di Studi in Dermatologia (GLISID). Bullous pemphigoid in Liguria: A 2-year survey. J Eur Acad Dermatol Venereol 2001;15: 317–319. [4] Gudi VS, White MI, Cruickshank N, Herriot R, Edwards SL, Nimmo F, Ormerod AD. Annual incidence and mortality of Update on pemphigoid [5] [6] [7] [8] Autoimmunity Downloaded from informahealthcare.com by Goteborgs University on 11/20/12 For personal use only. [9] [10] [11] [12] [13] [14] [15] [16] [17] [18] [19] bullous pemphigoid in the Grampian Region of North-east Scotland. Br J Dermatol 2005;153:424 –427. Serwin AB, Bokiniec E, Piascik M, Masny D, Chodynicka B. Epidemiological and clinical analysis of pemphigoid patients in northeastern Poland in 2000–2005. Med Sci Monit 2007; 13:CR360–CR364. Langan SM, Smeeth L, Hubbard R, Fleming KM, Smith CJ, West J. Bullous pemphigoid and pemphigus vulgaris— incidence and mortality in the UK: Population based cohort study. BMJ 2008;337:160–163. doi: 10.1136/bmj.a180. Schmidt E, Zillikens D. Modern diagnosis of autoimmune blistering skin diseases. Autoimmun Rev 2010;10:84–89. Stanley JR, Hawley-Nelson P, Yuspa SH, Shevach EM, Katz SI. Characterization of bullous pemphigoid antigen: A unique basement membrane protein of stratified epithelia. Cell 1981;24:897– 903. Mutasim DF, Takahashi Y, Labib RS, Anhalt GJ, Patel HP, Diaz LA. A pool of bullous pemphigoid antigen(s) is intracellular and associated with the basal cell cytoskeletonhemidesmosome complex. J Invest Dermatol 1985;84: 47–53. Labib RS, Anhalt GJ, Patel HP, Mutasim DF, Diaz LA. Molecular heterogeneity of bullous pemphigoid antigens as detected by immunoblotting. J Immunol 1986;136: 1231–1235. Tanaka M, Hashimoto T, Amagai M, Shimizu N, Ikeguchi N, Tsubata T, Hasegawa A, Miki K, Nishikawa T. Characterization of bullous pemphigoid antibodies by use of recombinant bullous pemphigoid antigen proteins. J Invest Dermatol 1991;97:725–728. Sawamura D, Li K, Chu ML, Uitto J, Human bullous pemphigoid antigen (BPAG1). Amino acid sequences deduced from cloned cDNAs predict biologically important peptide segments and protein domains. J Biol Chem 1991; 266:17784–17790. Green KJ, Virata ML, Elgart GW, Stanley JR, Parry DA. Comparative structural analysis of desmoplakin, bullous pemphigoid antigen and plectin: Members of a new gene family involved in organization of intermediate filaments. Int J Biol Macromol 1992;14:145–153. Giudice GJ, Emery DJ, Diaz LA. Cloning and primary structural analysis of the bullous pemphigoid autoantigen BP180. J Invest Dermatol 1992;99:243–250. Li K, Tamai K, Tan EM, Uitto J, Cloning of type XVII collagen. Complementary and genomic DNA sequences of mouse 180-kilodalton bullous pemphigoid antigen (BPAG2) predict an interrupted collagenous domain, a transmembrane segment, and unusual features in the 50 -end of the gene and the 30 -untranslated region of the mRNA. J Biol Chem 1993; 268:8825–8834. Giudice GJ, Emery DJ, Zelickson BD, Anhalt GJ, Liu Z, Diaz LA. Bullous pemphigoid and herpes gestationis autoantibodies recognize a common non-collagenous site on the BP180 ectodomain. J Immunol 1993;151:5742– 5750. Zillikens D, Rose PA, Balding SD, Liu Z, Olague-Marchan M, Diaz LA, Giudice GJ. Tight clustering of extracellular BP180 epitopes recognized by bullous pemphigoid autoantibodies. J Invest Dermatol 1997a;109:573–579. Zillikens D, Mascaro JM, Rose PA, Liu Z, Ewing SM, Caux F, Hoffmann RG, Diaz LA, Giudice GJ. A highly sensitive enzyme-linked immunosorbent assay for the detection of circulating anti-BP180 autoantibodies in patients with bullous pemphigoid. J Invest Dermatol 1997b;109:679 –683. Sitaru C, Dähnrich C, Probst C, Komorowski L, Blöcker I, Schmidt E, Schlumberger W, Rose C, Stöcker W, Zillikens D. Enzyme-linked immunosorbent assay using multimers of the 16th non-collagenous domain of the BP180 antigen for sensitive and specific detection of pemphigoid autoantibodies. Exp Dermatol 2007;16:770–777. 65 [20] Schmidt E, Obe K, Bröcker EB, Zillikens D. Serum levels of autoantibodies to BP180 correlate with disease activity in patients with bullous pemphigoid. Arch Dermatol 2000;136: 174 –178. [21] Zimina EP, Fritsch A, Schermer B, Bakulina AY, Bashkurov M, Benzing T, Bruckner-Tuderman L. Extracellular phosphorylation of collagen XVII by ectocasein kinase 2 inhibits ectodomain shedding. J Biol Chem 2007;282:22737 –22746. [22] Zimina EP, Hofmann SC, Fritsch A, Kern JS, Sitaru C, Bruckner-Tuderman L. Bullous pemphigoid autoantibodies preferentially recognize phosphoepitopes in collagen XVII. J Invest Dermatol 2008;128:2736–2739. [23] Leyendeckers H, Tasanen K, Bruckner-Tuderman L, Zillikens D, Sitaru C, Schmitz J, Hunzelmann N. Memory B cells specific for the NC16A domain of the 180 kDa bullous pemphigoid autoantigen can be detected in peripheral blood of bullous pemphigoid patients and induced in vitro to synthesize autoantibodies. J Invest Dermatol 2003;120: 372 –378. [24] László S, Neumann S, Hertl M, Hunzelmann N. Generation of increased numbers of HLA-DR(high) IgG þ plasma cells in the peripheral blood of patients with bullous pemphigoid: NC16a-specific cells belong to the short-lived plasma blast population. J Invest Dermatol 2010;130:2838–2841. [25] Bernard P, Aucouturier P, Denis F, Bonnetblanc JM. Immunoblot analysis of IgG subclasses of circulating antibodies in bullous pemphigoid. Clin Immunol Immunopathol 1990;54:484– 494. [26] Döpp R, Schmidt E, Chimanovitch I, Leverkus M, Bröcker EB, Zillikens D. IgG4 and IgE are the major immunoglobulins targeting the NC16A domain of BP180 in Bullous pemphigoid: Serum levels of these immunoglobulins reflect disease activity. J Am Acad Dermatol 2000;42:577–583. [27] Laffitte E, Skaria M, Jaunin F, Tamm K, Saurat JH, Favre B, Borradori L. Autoantibodies to the extracellular and intracellular domain of bullous pemphigoid 180, the putative key autoantigen in bullous pemphigoid, belong predominantly to the IgG1 and IgG4 subclasses. Br J Dermatol 2001; 144:760–768. [28] Dimson OG, Giudice GJ, Fu CL, Van den Bergh F, Warren SJ, Janson MM, Fairley JA. Identification of a potential effector function for IgE autoantibodies in the organ-specific autoimmune disease bullous pemphigoid. J Invest Dermatol 2003;120:784–788. [29] Iwata Y, Komura K, Kodera M, Usuda T, Yokoyama Y, Hara T, Muroi E, Ogawa F, Takenaka M, Sato S. Correlation of IgE autoantibody to BP180 with a severe form of bullous pemphigoid. Arch Dermatol 2008;144:41–48. [30] Fairley JA, Fu CL, Giudice GJ. Mapping the binding sites of anti-BP180 immunoglobulin E autoantibodies in bullous pemphigoid. J Invest Dermatol 2005;125:467– 472. [31] Giudice GJ, Wilske KC, Anhalt GJ, Fairley JA, Taylor AF, Emery DJ, Hoffman RG, Diaz LA. Development of an ELISA to detect anti-BP180 autoantibodies in bullous pemphigoid and herpes gestationis. J Invest Dermatol 1994; 102:878–881. [32] Perriard J, Jaunin F, Favre B, Büdinger L, Hertl M, Saurat JH, Borradori L. IgG autoantibodies from bullous pemphigoid (BP) patients bind antigenic sites on both the extracellular and the intracellular domains of the BP antigen 180. J Invest Dermatol 1999;112:141 –147. [33] Di Zenzo G, Grosso F, Terracina M, Mariotti F, De Pità O, Owaribe K, Mastrogiacomo A, Sera F, Borradori L, Zambruno G. Characterization of the anti-BP180 autoantibody reactivity profile and epitope mapping in bullous pemphigoid patients. J Invest Dermatol 2004;122:103–110. Autoimmunity Downloaded from informahealthcare.com by Goteborgs University on 11/20/12 For personal use only. 66 M. Kasperkiewicz et al. [34] Dresow SK, Sitaru C, Recke A, Oostingh GJ, Zillikens D, Gibbs BF. IgE autoantibodies against the intracellular domain of BP180. Br J Dermatol 2009;160:429–432. [35] Skaria M, Jaunin F, Hunziker T, Riou S, Schumann H, Bruckner-Tuderman L, Hertl M, Bernard P, Saurat JH, Favre B, Borradori L. IgG autoantibodies from bullous pemphigoid patients recognize multiple antigenic reactive sites located predominantly within the B and C subdomains of the COOH-terminus of BP230. J Invest Dermatol 2000; 114:998– 1004. [36] Kromminga A, Sitaru C, Hagel C, Herzog S, Zillikens D. Development of an ELISA for the detection of autoantibodies to BP230. Clin Immunol 2004;111:146 –152. [37] Thoma-Uszynski S, Uter W, Schwietzke S, Hofmann SC, Hunziker T, Bernard P, Treudler R, Zouboulis CC, Schuler G, Borradori L, Hertl M. BP230- and BP180-specific autoantibodies in bullous pemphigoid. J Invest Dermatol 2004; 122:1413–1422. [38] Di Zenzo G, Thoma-Uszynski S, Fontao L, Calabresi V, Hofmann SC, Hellmark T, Sebbag N, Pedicelli C, Sera F, Lacour JP, Wieslander J, Bruckner-Tuderman L, Borradori L, Zambruno G, Hertl M. Multicenter prospective study of the humoral autoimmune response in bullous pemphigoid. Clin Immunol 2008;128:415– 426. [39] Charneux J, Lorin J, Vitry F, Antonicelli F, Reguiai Z, Barbe C, Tabary T, Grange F, Bernard P. Usefulness of BP230 and BP180-NC16a enzyme-linked immunosorbent assays in the initial diagnosis of Bullous Pemphigoid: A retrospective study of 138 patients. Arch Dermatol 2011;147:286–291. [40] Roussel A, Benichou J, Randriamanantany ZA, Gilbert D, Drenovska K, Houivet E, Tron F, Joly P. Enzyme-linked immunosorbent assay for the combination of bullous pemphigoid antigens 1 and 2 in the diagnosis of bullous pemphigoid. Arch Dermatol 2011;147:293–298. [41] Thoma-Uszynski S, Uter W, Schwietzke S, Schuler G, Borradori L, Hertl M. Autoreactive T and B cells from bullous pemphigoid (BP) patients recognize epitopes clustered in distinct regions of BP180 and BP230. J Immunol 2015;176:2015–2023. [42] Büdinger L, Borradori L, Yee C, Eming R, Ferencik S, Grosse-Wilde H, Merk HF, Yancey K, Hertl M. Identification and characterization of autoreactive T cell responses to bullous pemphigoid antigen 2 in patients and healthy controls. J Clin Invest 1998;102:2082– 2089. [43] Lin MS, Fu CL, Giudice GJ, Olague-Marchan M, Lazaro AM, Stastny P, Diaz LA. Epitopes targeted by bullous pemphigoid T lymphocytes and autoantibodies map to the same sites on the bullous pemphigoid 180 ectodomain. J Invest Dermatol 2000;115:955 –961. [44] Schmidt E, Ambach A, Bastian B, Bröcker EB, Zillikens D. Elevated levels of interleukin-8 in blister fluid of bullous pemphigoid compared with suction blisters of healthy control subjects. J Am Acad Dermatol 1996;34:310–312. [45] Schmidt E, Bastian B, Dummer R, Tony HP, Bröcker EB, Zillikens D. Detection of elevated levels of IL-4, IL-6, and IL10 in blister fluid of bullous pemphigoid. Arch Dermatol Res 1996;288:353 –357. [46] Schmidt E, Mittnacht A, Schomig H, Dummer R, Bröcker EB, Zillikens D. Detection of IL-1 alpha, IL-1 beta and IL-1 receptor antagonist in blister fluid of bullous pemphigoid. J Dermatol Sci 1996;11:142–147. [47] Ameglio F, D’Auria L, Bonifati C, Ferraro C, Mastroianni A, Giacalone B. Cytokine pattern in blister fluid and serum of patients with bullous pemphigoid: Relationships with disease intensity. Br J Dermatol 1998;138:611 –614. [48] Inaoki M, Takehara K. Increased serum levels of interleukin (IL)-5, IL-6 and IL-8 in bullous pemphigoid. J Dermatol Sci 1998;16:152– 157. [49] D’Auria L, Bonifati C, Cordiali-Fei P, Leone G, Picardo M, Pietravalle M, Giacalone B, Ameglio F. Increased serum interleukin-15 levels in bullous skin diseases: Correlation with disease intensity. Arch Dermatol Res 1999;291:354–356. [50] Wakugawa W, Nakamura K, Hino H, Toyama K, Hattori N, Okochi H, Yamada H, Hirai K, Tamaki K, Furue M. Elevated levels of eotaxin and interleukin-5 in blister fluid of bullous pemphigoid: Correlation with tissue eosinophilia. Br J Dermatol 2000;143:112 –116. [51] Frezzolini A, Teofoli P, Cianchini G, Barduagni S, Ruffelli M, Ferranti G, Puddu P, Pita OD. Increased expression of eotaxin and its specific receptor CCR3 in bullous pemphigoid. Eur J Dermatol 2002;12:27–31. [52] Frezzolini A, Cianchini G, Ruffelli M, Cadoni S, Puddu P, De Pita O. Interleukin-16 expression and release in bullous pemphigoid. Clin Exp Immunol 2004;137:595– 600. [53] Gounni Abdelilah S, Wellemans V, Agouli M, Guenounou M, Hamid Q, Beck LA, Lamkhioued B. Increased expression of Th2-associated chemokines in bullous pemphigoid disease: Role of eosinophils in the production and release of these chemokines. Clin Immunol 2006;120:220–231. [54] Günther C, Carballido-Perrig N, Kopp T, Carballido JM, Pfeiffer C. CCL18 is expressed in patients with bullous pemphigoid and parallels disease course. Br J Dermatol 2009; 160:747–755. [55] Ludwig RJ, Schmidt E. Cytokines in autoimmune bullous skin diseases: Epiphenomena or contribution to pathogenesis? G Ital Dermatol Venereol 2009;144:339 –349. [56] Teraki Y, Hotta T, Shiohara T. Skin-homing interleukin-4 and -13-producing cells contribute to bullous pemphigoid: Remission of disease is associated with increased frequency of interleukin-10-producing cells. J Invest Dermatol 2001;117: 1097–1102. [57] Rensing-Ehl A, Gaus B, Bruckner-Tuderman L, Martin SF. Frequency, function and CLA expression of CD4 þ CD25 þ FOXP3 þ regulatory T cells in bullous pemphigoid. Exp Dermatol 2007;16:13 –21. [58] Oswald E, Fisch P, Jakob T, Bruckner-Tuderman L, Martin SF, Rensing-Ehl A. Reduced numbers of circulating gammadelta T cells in patients with bullous pemphigoid. Exp Dermatol 2009;18:991–993. [59] Kasperkiewicz M, Zillikens D. The pathophysiology of bullous pemphigoid. Clin Rev Allergy Immunol 2007;33: 67–77. [60] Bieber K, Sun S, Ishii N, Kasperkiewicz M, Schmidt E, Hirose M, Westermann J, Yu X, Zillikens D, Ludwig RJ. Animal models for autoimmune bullous dermatoses. Exp Dermatol 2010;19:2–11. [61] Schmidt E, Reimer S, Kruse N, Jainta S, Bröcker EB, Marinkovich MP, Giudice GJ, Zillikens D. Autoantibodies to BP180 associated with bullous pemphigoid release interleukin-6 and interleukin-8 from cultured human keratinocytes. J Invest Dermatol 2000;115:842 –848. [62] Iwata H, Kamio N, Aoyama Y, Yamamoto Y, Hirako Y, Owaribe K, Kitajima Y. IgG from patients with bullous pemphigoid depletes cultured keratinocytes of the 180-kDa bullous pemphigoid antigen (type XVII collagen) and weakens cell attachment. J Invest Dermatol 2009;129: 919 –926. [63] Gammon WR, Merritt CC, Lewis DM, Sams WM, Jr, Carlo JR, Wheeler CE, Jr. An in vitro model of immune complexmediated basement membrane zone separation caused by pemphigoid antibodies, leukocytes, and complement. J Invest Dermatol 1982;78:285–290. [64] Sitaru C, Schmidt E, Petermann S, Munteanu LS, Bröcker EB, Zillikens D. Autoantibodies to bullous pemphigoid antigen 180 induce dermal-epidermal separation in cryosections of human skin. J Invest Dermatol 2002;118:664 –671. Autoimmunity Downloaded from informahealthcare.com by Goteborgs University on 11/20/12 For personal use only. Update on pemphigoid [65] Liu Z, Diaz LA, Troy JL, Taylor AF, Emery DJ, Fairley JA, Giudice GJ. A passive transfer model of the organ-specific autoimmune disease, bullous pemphigoid, using antibodies generated against the hemidesmosomal antigen, BP180. J Clin Invest 1993;92:2480 –2488. [66] Liu Z, Giudice GJ, Swartz SJ, Fairley JA, Till GO, Troy JL, Diaz LA. The role of complement in experimental bullous pemphigoid. J Clin Invest 1995;95:1539 –1544. [67] Liu Z, Giudice GJ, Zhou X, Swartz SJ, Troy JL, Fairley JA, Till GO, Diaz LA. A major role for neutrophils in experimental bullous pemphigoid. J Clin Invest 1997;100: 1256–1263. [68] Chen R, Ning G, Zhao ML, Fleming MG, Diaz LA, Werb Z, Liu Z. Mast cells play a key role in neutrophil recruitment in experimental bullous pemphigoid. J Clin Invest 2001;108: 1151–1158. [69] Oikarinen AI, Zone JJ, Ahmed AR, Kiistala U, Uitto J. Demonstration of collagenase and elastase activities in the blister fluids from bullous skin diseases: Comparison between dermatitis herpetiformis and bullous pemphigoid. J Invest Dermatol 1983;81:261–266. [70] Welgus HG, Bauer EA, Stricklin GP. Elevated levels of human collagenase inhibitor in blister fluids of diverse etiology. J Invest Dermatol 1986;87:592– 596. [71] Jensen PJ, Baird J, Morioka S, Lessin S, Lazarus GS. Epidermal plasminogen activator is abnormal in cutaneous lesions. J Invest Dermatol 1988;90:777–782. [72] Grando SA, Glukhenky BT, Drannik GN, Kostromin AP, Chernyavsky AI. Cytotoxic proteases in blister fluid of pemphigus and pemphigoid patients. Int J Tissue React 1989; 11:195–201. [73] Grando SA, Glukhenky BT, Drannik GN, Epshtein EV, Kostromin AP, Korostash TA. Mediators of inflammation in blister fluids from patients with pemphigus vulgaris and bullous pemphigoid. Arch Dermatol 1989;125:925–930. [74] Gissler HM, Simon MM, Kramer MD. Enhanced association of plasminogen/plasmin with lesional epidermis of bullous pemphigoid. Br J Dermatol 1992;127:272–277. [75] Kramer MD, Reinartz J. The autoimmune blistering skin disease bullous pemphigoid: The presence of plasmin/alpha 2-antiplasmin complexes in skin blister fluid indicates plasmin generation in lesional skin. J Clin Invest 1993;92: 978–983. [76] Ståhle-Bäckdahl M, Inoue M, Guidice GJ, Parks WC. 92-kD gelatinase is produced by eosinophils at the site of blister formation in bullous pemphigoid and cleaves the extracellular domain of recombinant 180-kD bullous pemphigoid autoantigen. J Clin Invest 1994;93:2022–2030. [77] Niimi Y, Pawankar R, Kawana S. Increased expression of matrix metalloproteinase-2, matrix metalloproteinase-9 and matrix metalloproteinase-13 in lesional skin of bullous pemphigoid. Int Arch Allergy Immunol 2006;139: 104–113. [78] Verraes S, Hornebeck W, Polette M, Borradori L, Bernard P. Respective contribution of neutrophil elastase and matrix metalloproteinase 9 in the degradation of BP180 (type XVII collagen) in human bullous pemphigoid. J Invest Dermatol 2001;117:1091–1096. [79] Shimanovich I, Mihai S, Oostingh GJ, Ilenchuk TT, Bröcker EB, Opdenakker G, Zillikens D, Sitaru C. Granulocyte-derived elastase and gelatinase B are required for dermal-epidermal separation induced by autoantibodies from patients with epidermolysis bullosa acquisita and bullous pemphigoid. J Pathol 2004;204:519–527. [80] Liu Z, Shipley JM, Vu TH, Zhou X, Diaz LA, Werb Z, Senior RM. Gelatinase B-deficient mice are resistant to experimental bullous pemphigoid. J Exp Med 1998;188:475–482. [81] Liu Z, Shapiro SD, Zhou X, Twining SS, Senior RM, Giudice GJ, Fairley JA, Diaz LA. A critical role for neutrophil [82] [83] [84] [85] [86] [87] [88] [89] [90] [91] [92] [93] [94] [95] 67 elastase in experimental bullous pemphigoid. J Clin Invest 2000;105:113–123. Liu Z, Li N, Diaz LA, Shipley M, Senior RM, Werb Z. Synergy between a plasminogen cascade and MMP-9 in autoimmune disease. J Clin Invest 2005;115:879 –887. Schmidt E, Wehr B, Tabengwa EM, Reimer S, Bröcker EB, Zillikens D. Elevated expression and release of tissue-type, but not urokinase-type, plasminogen activator after binding of autoantibodies to bullous pemphigoid antigen 180 in cultured human keratinocytes. Clin Exp Immunol 2004;135: 497 –504. Coussens LM, Raymond WW, Bergers G, Laig-Webster M, Behrendtsen O, Werb Z, Caughey GH, Hanahan D. Inflammatory mast cells up-regulate angiogenesis during squamous epithelial carcinogenesis. Genes Dev 1999;13: 1382–1397. Liu Z, Zhou X, Shapiro SD, Shipley JM, Twining SS, Diaz LA, Senior RM, Werb Z. The serpin alpha1-proteinase inhibitor is a critical substrate for gelatinase B/MMP-9 in vivo. Cell 2000;102:647–655. Zillikens D, Schmidt E, Reimer S, Chimanovitch I, Hardt-Weinelt K, Rose C, Bröcker EB, Kock M, Boehncke WH. Antibodies to desmogleins 1 and 3, but not to BP180, induce blisters in human skin grafted onto SCID mice. J Pathol 2001;193:117– 124. Olasz EB, Roh J, Yee CL, Arita K, Akiyama M, Shimizu H, Vogel JC, Yancey KB. Human bullous pemphigoid antigen 2 transgenic skin elicits specific IgG in wild-type mice. J Invest Dermatol 2007;127:2807–2817. Nishie W, Sawamura D, Goto M, Ito K, Shibaki A, McMillan JR, Sakai K, Nakamura H, Olasz E, Yancey KB, Akiyama M, Shimizu H. Humanization of autoantigen. Nat Med 2007;13:378–383. Li Q, Ujiie H, Shibaki A, Wang G, Moriuchi R, Qiao HJ, Morioka H, Shinkuma S, Natsuga K, Long HA, Nishie W, Shimizu H. Human IgG1 monoclonal antibody against human collagen 17 NC16A induces blisters via complement activation in experimental bullous pemphigoid model. J Immunol 2010;185:7746–7755. Liu Z, Sui W, Zhao M, Li Z, Li N, Thresher R, Giudice GJ, Fairley JA, Sitaru C, Zillikens D, Ning G, Marinkovich MP, Diaz LA. Subepidermal blistering induced by human autoantibodies to BP180 requires innate immune players in a humanized bullous pemphigoid mouse model. J Autoimmun 2008;31:331–338. Ujiie H, Shibaki A, Nishie W, Sawamura D, Wang G, Tateishi Y, Li Q, Moriuchi R, Qiao H, Nakamura H, Akiyama M, Shimizu H. A novel active mouse model for bullous pemphigoid targeting humanized pathogenic antigen. J Immunol 2010;184:2166–2174. Zone JJ, Taylor T, Hull C, Schmidt L, Meyer L. IgE basement membrane zone antibodies induce eosinophil infiltration and histological blisters in engrafted human skin on SCID mice. J Invest Dermatol 2007;127:1167–1174. Fairley JA, Burnett CT, Fu CL, Larson DL, Fleming MG, Giudice GJ. A pathogenic role for IgE in autoimmunity: Bullous pemphigoid IgE reproduces the early phase of lesion development in human skin grafted to nu/nu mice. J Invest Dermatol 2007;127:2605–2611. Joly P, Benichou J, Lok C, Hellot MF, Saiag P, TancredeBohin E, Sassolas B, Labeille B, Doutre MS, Gorin I, Pauwels C, Chosidow O, Caux F, Estève E, Dutronc Y, Sigal M, Prost C, Maillard H, Guillaume JC, Roujeau JC. Prediction of survival for patients with bullous pemphigoid: A prospective study. Arch Dermatol 2005;141:691 –698. Parker SR, Dyson S, Brisman S, Pennie M, Swerlick RA, Khan R, Manos S, Korman BD, Xia Z, Korman NJ. Mortality of bullous pemphigoid: An evaluation of 223 patients and comparison with the mortality in the general 68 [96] [97] [98] Autoimmunity Downloaded from informahealthcare.com by Goteborgs University on 11/20/12 For personal use only. [99] [100] [101] [102] [103] [104] [105] [106] [107] [108] M. Kasperkiewicz et al. population in the United States. J Am Acad Dermatol 2008; 59:582–588. Kelly SE, Wojnarowska F. The use of chemically split tissue in the detection of circulating anti-basement membrane zone antibodies in bullous pemphigoid and cicatricial pemphigoid. Br J Dermatol 1988;118:31–40. Kobayashi M, Amagai M, Kuroda-Kinoshita K, Hashimoto T, Shirakata Y, Hashimoto K, Nishikawa T. BP180 ELISA using bacterial recombinant NC16a protein as a diagnostic and monitoring tool for bullous pemphigoid. J Dermatol Sci 2002;30:224– 232. Hofmann S, Thoma-Uszynski S, Hunziker T, Bernard P, Koebnick C, Stauber A, Schuler G, Borradori L, Hertl M. Severity and phenotype of bullous pemphigoid relate to autoantibody profile against the NH2- and COOH-terminal regions of the BP180 ectodomain. J Invest Dermatol 2002; 119:1065–1073. Yoshida M, Hamada T, Amagai M, Hashimoto K, Uehara R, Yamaguchi K, Imamura K, Okamoto E, Yasumoto S, Hashimoto T. Enzyme-linked immunosorbent assay using bacterial recombinant proteins of human BP230 as a diagnostic tool for bullous pemphigoid. J Dermatol Sci 2006;41:21–30. Vodegel RM, Jonkman MF, Pas HH, de Jong MC. U-serrated immunodeposition pattern differentiates type VII collagen targeting bullous diseases from other subepidermal bullous autoimmune diseases. Br J Dermatol 2004; 151:112–118. Roujeau JC, Lok C, Bastuji-Garin S, Mhalla S, Enginger V, Bernard P. High risk of death in elderly patients with extensive bullous pemphigoid. Arch Dermatol 1998;134: 465 –469. Rzany B, Partscht K, Jung M, Kippes W, Mecking D, Baima B, Prudlo C, Pawelczyk B, Messmer EM, Schuhmann M, Sinkgraven R, Büchner L, Büdinger L, Pfeiffer C, Sticherling M, Hertl M, Kaiser HW, Meurer M, Zillikens D, Messer G. Risk factors for lethal outcome in patients with bullous pemphigoid: Low serum albumin level, high dosage of glucocorticosteroids, and old age. Arch Dermatol 2002;138: 903 –908. Colbert RL, Allen DM, Eastwood D, Fairley JA. Mortality rate of bullous pemphigoid in a US medical center. J Invest Dermatol 2004;122:1091–1095. Kirtschig G, Middleton P, Bennett C, Murrell DF, Wojnarowska F, Khumalo NP. Interventions for bullous pemphigoid. Cochrane Database Syst Rev 2010;CD002292. Morel P, Guillaume JC. Treatment of bullous pemphigoid with prednisolone only: 0.75 mg/kg/day versus 1.25 mg/kg/ day. A multicenter randomized study. Ann Dermatol Venereol 1984;111:925 –928. Joly P, Roujeau JC, Benichou J, Picard C, Dreno B, Delaporte E, Vaillant L, D’Incan M, Plantin P, Bedane C, Young P, Bernard P, Bullous Diseases French Study Group. A comparison of oral and topical corticosteroids in patients with bullous pemphigoid. N Engl J Med 2002;346:321 –327. Joly P, Roujeau JC, Benichou J, Delaporte E, D’Incan M, Dreno B, Bedane C, Sparsa A, Gorin I, Picard C, TancredeBohin E, Sassolas B, Lok C, Guillaume JC, Doutre MS, Richard MA, Caux F, Prost C, Plantin P, Chosidow O, Pauwels C, Maillard H, Saiag P, Descamps V, ChevrantBreton J, Dereure O, Hellot MF, Esteve E, Bernard P. A comparison of two regimens of topical corticosteroids in the treatment of patients with bullous pemphigoid: A multicenter randomized study. J Invest Dermatol 2009 July;129(7): 1681–1687. Guillaume JC, Vaillant L, Bernard P, Picard C, Prost C, Labeille B, Guillot B, Foldès-Pauwels C, Prigent F, Joly P, Cordoliani F, Salagnac V, Machet L, Aldigier JC, Crickx B, Roujeau JC. Controlled trial of azathioprine and plasma [109] [110] [111] [112] [113] [114] [115] [116] [117] [118] [119] [120] [121] [122] [123] exchange in addition to prednisolone in the treatment of bullous pemphigoid. Arch Dermatol 1993;129:49– 53. Beissert S, Werfel T, Frieling U, Böhm M, Sticherling M, Stadler R, Zillikens D, Rzany B, Hunzelmann N, Meurer M, Gollnick H, Ruzicka T, Pillekamp H, Junghans V, Bonsmann G, Luger TA. A comparison of oral methylprednisolone plus azathioprine or mycophenolate mofetil for the treatment of bullous pemphigoid. Arch Dermatol 2007;143:1536–1542. Fivenson DP, Breneman DL, Rosen GB, Hersh CS, Cardone S, Mutasim D. Nicotinamide and tetracycline therapy of bullous pemphigoid. Arch Dermatol 1994;130:753 –758. Schmidt E, Kraensel R, Goebeler M, Sinkgraven R, Bröcker EB, Rzany B, Zillikens D. Treatment of bullous pemphigoid with dapsone, methylprednisolone, and topical clobetasol propionate: A retrospective study of 62 cases. Cutis 2005;76: 205–209. Gürcan HM, Ahmed AR. Efficacy of dapsone in the treatment of pemphigus and pemphigoid: Analysis of current data. Am J Clin Dermatol 2009;10:383– 396. Gürcan HM, Ahmed AR. Analysis of current data on the use of methotrexate in the treatment of pemphigus and pemphigoid. Br J Dermatol 2009;161:723 –731. Ishii N, Hashimoto T, Zillikens D, Ludwig RJ. High-dose intravenous immunoglobulin (IVIG) therapy in autoimmune skin blistering diseases. Clin Rev Allergy Immunol 2010;38: 186–195. Schmidt E, Bröcker EB, Goebeler M. Rituximab in treatment-resistant autoimmune blistering skin disorders. Clin Rev Allergy Immunol 2008;34:56–64. Hertl M, Zillikens D, Borradori L, Bruckner-Tuderman L, Burckhard H, Eming R, Engert A, Goebeler M, Hofmann S, Hunzelmann N, Karlhofer F, Kautz O, Lippert U, Niedermeier A, Nitschke M, Pfütze M, Reiser M, Rose C, Schmidt E, Shimanovich I, Sticherling M, Wolff-Franke S. Recommendations for the use of rituximab (anti-CD20 antibody) in the treatment of autoimmune bullous skin diseases. J Dtsch Dermatol Ges 2008;6:366–373. Kasperkiewicz M, Shimanovich I, Ludwig RJ, Rose C, Zillikens D, Schmidt E. Rituximab for treatment-refractory pemphigus and pemphigoid: A case series of 17 patients. J Am Acad Dermatol 2011;65:552–558. Fairley JA, Baum CL, Brandt DS, Messingham KA. Pathogenicity of IgE in autoimmunity: Successful treatment of bullous pemphigoid with omalizumab. J Allergy Clin Immunol 2009;123:704–705. Schmidt E, Zillikens D. Immunoadsorption in dermatology. Arch Dermatol Res 2010;302:241–253. Schmidt E, Skrobek C, Kromminga A, Hashimoto T, Messer G, Bröcker EB, Yancey KB, Zillikens D. Cicatricial pemphigoid: IgA and IgG autoantibodies target epitopes on both intra- and extracellular domains of bullous pemphigoid antigen 180. Br J Dermatol 2001;145:778 –783. Bédane C, McMillan JR, Balding SD, Bernard P, Prost C, Bonnetblanc JM, Diaz LA, Eady RA, Giudice GJ. Bullous pemphigoid and cicatricial pemphigoid autoantibodies react with ultrastructurally separable epitopes on the BP180 ectodomain: Evidence that BP180 spans the lamina lucida. J Invest Dermatol 1997;108:901–907. Oyama N, Setterfield JF, Powell AM, Sakuma-Oyama Y, Albert S, Bhogal BS, Vaughan RW, Kaneko F, Challacombe SJ, Black MM. Bullous pemphigoid antigen II (BP180) and its soluble extracellular domains are major autoantigens in mucous membrane pemphigoid: The pathogenic relevance to HLA class II alleles and disease severity. Br J Dermatol 2006; 154:90– 98. Bernard P, Prost C, Lecerf V, Intrator L, Combemale P, Bedane C, Roujeau JC, Revuz J, Bonnetblanc JM, Dubertret L. Studies of cicatricial pemphigoid autoantibodies using Update on pemphigoid [124] [125] [126] Autoimmunity Downloaded from informahealthcare.com by Goteborgs University on 11/20/12 For personal use only. [127] [128] [129] [130] [131] [132] [133] [134] [135] [136] [137] [138] direct immunoelectron microscopy and immunoblot analysis. J Invest Dermatol 1990;94:630–635. Calabresi V, Carrozzo M, Cozzani E, Arduino P, Bertolusso G, Tirone F, Parodi A, Zambruno G, Di Zenzo G. Oral pemphigoid autoantibodies preferentially target BP180 ectodomain. Clin Immunol 2007;122:207 –213. Domloge-Hultsch N, Gammon WR, Briggaman RA, Gil SG, Carter WG, Yancey KB. Epiligrin, the major human keratinocyte integrin ligand, is a target in both an acquired autoimmune and an inherited subepidermal blistering skin disease. J Clin Invest 1992;90:1628–1633. Chan LS, Majmudar AA, Tran HH, Meier F, SchaumburgLever G, Chen M, Anhalt G, Woodley DT, Marinkovich MP. Laminin-6 and laminin-5 are recognized by autoantibodies in a subset of cicatricial pemphigoid. J Invest Dermatol 1997; 108:848–853. Luke MC, Darling TN, Hsu R, Summers RM, Smith JA, Solomon BI, Thomas GR, Yancey KB. Mucosal morbidity in patients with epidermolysis bullosa acquisita. Arch Dermatol 1999;135:954–959. Tyagi S, Bhol K, Natarajan K, Livir-Rallatos C, Foster CS, Ahmed AR. Ocular cicatricial pemphigoid antigen: Partial sequence and biochemical characterization. Proc Natl Acad Sci USA 1996;93:14714 –14719. Rashid KA, Gürcan HM, Ahmed AR. Antigen specificity in subsets of mucous membrane pemphigoid. J Invest Dermatol 2006;126:2631 –2636. Lazarova Z, Yee C, Darling T, Briggaman RA, Yancey KB. Passive transfer of anti-laminin 5 antibodies induces subepidermal blisters in neonatal mice. J Clin Invest 1996; 98:1509–1518. Lazarova Z, Hsu R, Briggaman RA, Yancey KB. Fab fragments directed against laminin 5 induce subepidermal blisters in neonatal mice. Clin Immunol 2000;95:26–32. Lazarova Z, Hsu R, Yee C, Yancey KB. Human anti-laminin 5 autoantibodies induce subepidermal blisters in an experimental human skin graft model. J Invest Dermatol 2000;114:178–184. Razzaque MS, Foster CS, Ahmed AR. Tissue and molecular events in human conjunctival scarring in ocular cicatricial pemphigoid. Histol Histopathol 2001;16:1203– 1212. Saw VP, Schmidt E, Offiah I, Galatowicz G, Zillikens D, Dart JK, Calder VL, Daniels JT. Profibrotic phenotype of conjunctival fibroblasts from mucous membrane pemphigoid. Am J Pathol 2011;178:187– 197. Chan LS, Ahmed AR, Anhalt GJ, Bernauer W, Cooper KD, Elder MJ, Fine JD, Foster CS, Ghohestani R, Hashimoto T, Hoang-Xuan T, Kirtschig G, Korman NJ, Lightman S, Lozada-Nur F, Marinkovich MP, Mondino BJ, ProstSquarcioni C, Rogers RS, 3rd, Setterfield JF, West DP, Wojnarowska F, Woodley DT, Yancey KB, Zillikens D, Zone JJ. The first international consensus on mucous membrane pemphigoid: Definition, diagnostic criteria, pathogenic factors, medical treatment, and prognostic indicators. Arch Dermatol 2002;138:370 –379. Rose C, Schmidt E, Kerstan A, Thoma-Uszynski S, Wesselmann U, Käsbohrer U, Zillikens D, Shimanovich I. Histopathology of anti-laminin 5 mucous membrane pemphigoid. J Am Acad Dermatol 2009;61:433–440. Horváth B, Niedermeier A, Podstawa E, Müller R, Hunzelmann N, Kárpáti S, Hertl M. IgA autoantibodies in the pemphigoids and linear IgA bullous dermatosis. Exp Dermatol 2010;19:648–653. Leverkus M, Schmidt E, Lazarova Z, Bröcker EB, Yancey KB, Zillikens D. Antiepiligrin cicatricial pemphigoid: An underdiagnosed entity within the spectrum of scarring autoimmune subepidermal bullous diseases? Arch Dermatol 1999;135:1091 –1098. 69 [139] Egan CA, Lazarova Z, Darling TN, Yee C, Coté T, Yancey KB. Anti-epiligrin cicatricial pemphigoid and relative risk for cancer. Lancet 2001;357:1850–1851. [140] Lazarova Z, Sitaru C, Zillikens D, Yancey KB. Comparative analysis of methods for detection of anti-laminin 5 autoantibodies in patients with anti-epiligrin cicatricial pemphigoid. J Am Acad Dermatol 2004;51:886–892. [141] Brunsting LA, Perry HO. Benign pemphigoid? A report of seven cases with chronic, scarring, herpetiform plaques about the head and neck. Arch Dermatol 1957;75:489–501. [142] Kurzhals G, Stolz W, Meurer M, Kunze J, Braun-Falco O, Krieg T. Acquired epidermolysis bullosa with the clinical feature of Brunsting –Perry cicatricial bullous pemphigoid. Arch Dermatol 1991;127:391–395. [143] Daito J, Katoh N, Asai J, Ueda E, Takenaka H, Ishii N, Hashimoto T, Kishimoto S. Brunsting–Perry cicatricial pemphigoid associated with autoantibodies to the C-terminal domain of BP180. Br J Dermatol 2008;159:984 –986. [144] Jedlickova H, Niedermeier A, Zgažarová S, Hertl M. Brunsting–Perry pemphigoid of the scalp with antibodies against laminin 332. Dermatology 2011;222:193 –195. [145] Meyer-ter-Vehn T, Schmidt E, Zillikens D, Geerling G. Mucous membrane pemphigoid with ocular involvement. Part II: Therapy. Ophthalmologe 2008;105:405 –419. [146] Knudson RM, Kalaaji AN, Bruce AJ. The management of mucous membrane pemphigoid and pemphigus. Dermatol Ther 2010;23:268–280. [147] Sami N, Letko E, Androudi S, Daoud Y, Foster CS, Ahmed AR. Intravenous immunoglobulin therapy in patients with ocular-cicatricial pemphigoid: A long-term follow-up. Ophthalmology 2004;111:1380– 1382. [148] O’Neill ID. Off-label use of biologicals in the management of inflammatory oral mucosal disease. J Oral Pathol Med 2008; 37:575– 581. [149] Shimanovich I, Bröcker EB, Zillikens D. Pemphigoid gestationis: New insights into the pathogenesis lead to novel diagnostic tools. BJOG 2002;109:970–976. [150] Morrison LH, Labib RS, Zone JJ, Diaz LA, Anhalt GJ. Herpes gestationis autoantibodies recognize a 180-kD human epidermal antigen. J Clin Invest 1988;81:2023– 2026. [151] Murakami H, Amagai M, Higashiyama M, Hashimoto K, Chorzelski TP, Bhogal BS, Jenkins RE, Black MM, Zillikens D, Nishikawa T, Hashimoto T. Analysis of antigens recognized by autoantibodies in herpes gestationis: Usefulness of immunoblotting using a fusion protein representing an extracellular domain of the 180 kD bullous pemphigoid antigen. J Dermatol Sci 1996;13:112–117. [152] Chimanovitch I, Schmidt E, Messer G, Döpp R, Partscht K, Bröcker EB, Giudice GJ, Zillikens D. IgG1 and IgG3 are the major immunoglobulin subclasses targeting epitopes within the NC16A domain of BP180 in pemphigoid gestationis. J Invest Dermatol 1999;113:140–142. [153] Di Zenzo G, Calabresi V, Grosso F, Caproni M, Ruffelli M, Zambruno G. The intracellular and extracellular domains of BP180 antigen comprise novel epitopes targeted by pemphigoid gestationis autoantibodies. J Invest Dermatol 2007;127:864–873. [154] Herrero-González JE, Brauns O, Egner R, Rönspeck W, Mascaró JM, Jr, Jonkman MF, Zillikens D, Sitaru C. Immunoadsorption against two distinct epitopes on human type XVII collagen abolishes dermal-epidermal separation induced in vitro by autoantibodies from pemphigoid gestationis patients. Eur J Immunol 2006;36:1039–1048. [155] Döpp R, Schmidt E, Chimanovitch I, Leverkus M, Bröcker EB, Zillikens D. IgG4 and IgE are the major immunoglobulins targeting the NC16A domain of BP180 in bullous pemphigoid: Serum levels of these immunoglobulins reflect disease activity. J Am Acad Dermatol 2000;42:577–583. Autoimmunity Downloaded from informahealthcare.com by Goteborgs University on 11/20/12 For personal use only. 70 M. Kasperkiewicz et al. [156] Lin MS, Gharia MA, Swartz SJ, Diaz LA, Giudice GJ. Identification and characterization of epitopes recognized by T lymphocytes and autoantibodies from patients with herpes gestationis. J Immunol 1999;162:4991–4997. [157] Sitaru C, Powell J, Messer G, Bröcker EB, Wojnarowska F, Zillikens D. Immunoblotting and enzyme-linked immunosorbent assay for the diagnosis of pemphigoid gestationis. Obstet Gynecol 2004;103:757– 763. [158] Wojnarowska F, Whitehead P, Leigh IM, Bhogal BS, Black MM. Identification of the target antigen in chronic bullous disease of childhood and linear IgA disease of adults. Br J Dermatol 1991;124:157–162. [159] Marinkovich MP, Taylor TB, Keene DR, Burgeson RE, Zone JJ. LAD-1, the linear IgA bullous dermatosis autoantigen, is a novel 120-kDa anchoring filament protein synthesized by epidermal cells. J Invest Dermatol 1996;106: 734 –738. [160] Zone JJ, Taylor TB, Meyer LJ, Petersen MJ. The 97 kDa linear IgA bullous disease antigen is identical to a portion of the extracellular domain of the 180 kDa bullous pemphigoid antigen, BPAg2. J Invest Dermatol 1998;110:207 –210. [161] Zone JJ, Taylor TB, Kadunce DP, Meyer LJ. Identification of the cutaneous basement membrane zone antigen and isolation of antibody in linear immunoglobulin A bullous dermatosis. J Clin Invest 1990;85:812–820. [162] Pas HH, Kloosterhuis GJ, Heeres K, van der Meer JB, Jonkman MF. Bullous pemphigoid and linear IgA dermatosis sera recognize a similar 120-kDa keratinocyte collagenous glycoprotein with antigenic cross-reactivity to BP180. J Invest Dermatol 1997;108:423 –429. [163] Hirako Y, Usukura J, Uematsu J, Hashimoto T, Kitajima Y, Owaribe K. Cleavage of BP180, a 180-kDa bullous pemphigoid antigen, yields a 120-kDa collagenous extracellular polypeptide. J Biol Chem 1998;273:9711–9717. [164] Schäcke H, Schumann H, Hammami-Hauasli N, Raghunath M, Bruckner-Tuderman L. Two forms of collagen XVII in keratinocytes: A full-length transmembrane protein and a soluble ectodomain. J Biol Chem 1998;273:25937–25943. [165] Hirako Y, Nishizawa Y, Sitaru C, Opitz A, Marcus K, Meyer HE, Butt E, Owaribe K, Zillikens D. The 97-kDa (LABD97) and 120-kDa (LAD-1) fragments of bullous pemphigoid antigen 180/type XVII collagen have different N-termini. J Invest Dermatol 2003;121:1554–1556. [166] Franzke CW, Bruckner-Tuderman L, Blobel CP. Shedding of collagen XVII/BP180 in skin depends on both ADAM10 and ADAM9. J Biol Chem 2009;284:23386 –23396. [167] Hofmann SC, Voith U, Schönau V, Sorokin L, BrucknerTuderman L, Franzke CW. Plasmin plays a role in the in vitro generation of the linear IgA dermatosis antigen LADB97. J Invest Dermatol 2009;129:1730–1739. [168] Zillikens D, Herzele K, Georgi M, Schmidt E, Chimanovitch I, Schumann H, Mascaro JM, Jr, Diaz LA, Bruckner-Tuderman L, Bröcker EB, Giudice GJ. Autoantibodies in a subgroup of patients with linear IgA disease react with the NC16A domain of BP180. J Invest Dermatol 1999a;113:947–953. [169] Zambruno G, Manca V, Kanitakis J, Cozzani E, Nicolas JF, Giannetti A. Linear IgA bullous dermatosis with autoantibodies to a 290 kDa antigen of anchoring fibrils. J Am Acad Dermatol 1994;31:884– 888. [170] Ghohestani RF, Nicolas JF, Kanitakis J, Claudy A. Linear IgA bullous dermatosis with IgA antibodies exclusively directed against the 180- or 230-kDa epidermal antigens. J Invest Dermatol 1997;108:854 –858. [171] Natsuga K, Nishie W, Shinkuma S, Moriuchi R, Shibata M, Nishimura M, Hashimoto T, Shimizu H. Circulating IgA and IgE autoantibodies in antilaminin-332 mucous membrane pemphigoid. Br J Dermatol 2010;162:513– 517. [172] Kromminga A, Scheckenbach C, Georgi M, Hagel C, Arndt R, Christophers E, Bröcker EB, Zillikens D. Patients with [173] [174] [175] [176] [177] [178] [179] [180] [181] [182] [183] [184] [185] [186] [187] [188] [189] bullous pemphigoid and linear IgA disease show a dual IgA and IgG autoimmune response to BP180. J Autoimmun 2000;15:293–300. Mulyowa GK, Jaeger G, Kabakyenga J, Bröcker EB, Zillikens D, Schmidt E. Autoimmune subepidermal blistering diseases in Uganda: Correlation of autoantibody class with age of patients. Int J Dermatol 2006;45:1047–1052. Zone JJ, Egan CA, Taylor TB, Meyer LJ. IgA autoimmune disorders: Development of a passive transfer mouse model. J Investig Dermatol Symp Proc 2004;9:47–51. Guide SV, Marinkovich MP. Linear IgA bullous dermatosis. Clin Dermatol 2001;19:719– 727. Kasperkiewicz M, Meier M, Zillikens D, Schmidt E. Linear IgA disease: Successful application of immunoadsorption and review of the literature. Dermatology 2010;220: 259–263. Willsteed E, Bhogal BS, Das AK, Wojnarowska F, Black MM, McKee PH. Lichen planus pemphigoides: A clinicopathological study of nine cases. Histopathology 1991;19:147– 154. Davis AL, Bhogal BS, Whitehead P, Frith P, Murdoch ME, Leigh IM, Wojnarowska F. Lichen planus pemphigoides: Its relationship to bullous pemphigoid. Br J Dermatol 1991;125: 263–271. Bouloc A, Vignon-Pennamen MD, Caux F, Teillac D, Wechsler J, Heller M, Lebbé C, Flageul B, Morel P, Dubertret L, Prost C. Lichen planus pemphigoides is a heterogeneous disease: A report of five cases studied by immunoelectron microscopy. Br J Dermatol 1998;138: 972–980. Zillikens D, Caux F, Mascaro JM, Wesselmann U, Schmidt E, Prost C, Callen JP, Bröcker EB, Diaz LA, Giudice GJ. Autoantibodies in lichen planus pemphigoides react with a novel epitope within the C-terminal NC16A domain of BP180. J Invest Dermatol 1999b;113:117 –121. Zillikens D, Kawahara Y, Ishiko A, Shimizu H, Mayer J, Rank CV, Liu Z, Giudice GJ, Tran HH, Marinkovich MP, Brocker EB, Hashimoto T. A novel subepidermal blistering disease with autoantibodies to a 200-kDa antigen of the basement membrane zone. J Invest Dermatol 1996;106:1333 –1338. Dainichi T, Kurono S, Ohyama B, Ishii N, Sanzen N, Hayashi M, Shimono C, Taniguchi Y, Koga H, Karashima T, Yasumoto S, Zillikens D, Sekiguchi K, Hashimoto T. Antilaminin gamma-1 pemphigoid. Proc Natl Acad Sci U S A 2009;106:2800–2805. Groth S, Recke A, Vafia K, Ludwig RJ, Hashimoto T, Zillikens D, Schmidt E. Development of a simple enzymelinked immunosorbent assay for the detection of autoantibodies in anti-p200 pemphigoid. Br J Dermatol 2011;164: 76– 82. Timpl R, Rohde H, Robey PG, Rennard SI, Foidart JM, Martin GR. Laminin — A glycoprotein from basement membranes. J Biol Chem 1979;254:9933–9937. Dainichi T, Koga H, Tsuji T, Ishii N, Ohyama B, Ueda A, Natsuaki Y, Karashima T, Nakama T, Yasumoto S, Zillikens D, Hashimoto T. From anti-p200 pemphigoid to antilaminin gamma1 pemphigoid. J Dermatol 2010;37:231–238. Iwata H, Hiramitsu Y, Aoyama Y, Kitajima Y. A case of antip200 pemphigoid: Evidence for a different pathway in neutrophil recruitment compared with bullous pemphigoid. Br J Dermatol 2009;160:462 –464. Dilling A, Rose C, Hashimoto T, Zillikens D, Shimanovich I. Anti-p200 pemphigoid: A novel autoimmune subepidermal blistering disease. J Dermatol 2007;34:1–8. Rose C, Weyers W, Denisjuk N, Hillen U, Zillikens D, Shimanovich I. Histopathology of anti-p200 pemphigoid. Am J Dermatopathol 2007;29:119–124. Kasperkiewicz M, Hoppe U, Zillikens D, Schmidt E. Relapse-associated autoantibodies to BP180 in a patient with anti-p200 pemphigoid. Clin Exp Dermatol 2010;35:614.