* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Electrostatics
Introduction to gauge theory wikipedia , lookup
Maxwell's equations wikipedia , lookup
Electron mobility wikipedia , lookup
Magnetic monopole wikipedia , lookup
Nuclear physics wikipedia , lookup
History of subatomic physics wikipedia , lookup
Electromagnetism wikipedia , lookup
Fundamental interaction wikipedia , lookup
Electrical resistivity and conductivity wikipedia , lookup
Lorentz force wikipedia , lookup
Atomic nucleus wikipedia , lookup
Elementary particle wikipedia , lookup
Atomic theory wikipedia , lookup
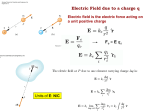
Electrostatics What is Electrostatics? Electrostatics is the study of the interactions between stationary electrically charged particles. Electrostatic laws deal with the attractive and repelling forces that exist between positive and negative electric charges. Four Fundamental Forces Strong Force Electromagnetic Force Weak Nuclear Force Gravitational Force https://www.youtube.com/watch?v=5HLTHrcnO74 https://www.youtube.com/watch?v=U0kXkWXSXRA&app=desktop SI unit of Charge: the Coulomb • SI unit for charge is the coulomb, and its symbol is C. • A coulomb is a fairly large amount of charge, so sometimes we measure small amounts of charge in μC • An electron has a charge of -1.6 10-19 C. • A proton has a charge of +1.6 10-19 C. • In a wire, if one coulomb of charge flows past a point in one second, we say the current in the wire is one ampere. Elementary Charge • Charges come in small, discrete bundles (quantized). This means an object can possess charge in incremental, rather than continuous, amounts. • The smallest amount of charge that can be added or removed from an object is the elementary charge, e = 1.6 10-19 C. • The charge of a proton is +e, an electron -e. • The charge of an object, Q, is always a multiple of this elementary charge: Q = Ne, where N is an integer. What is Net Charge? Net charge is the amount of excess charge; a neutral object has an equal number of electrons and protons, and therefore, no net charge. No Net Charge Positive Net Charge A negatively charged object has: (a) Only positive charges. (b) Only negative charges. (c) More positive charges than negative. (d) More negative charges than positive. A negatively charged object has: (d) More negative charges than positive. JUST BECAUSE AN OBJECT IS NEGATIVELY CHARGED DOES NOT MEAN IT HAS NO PROTONS . • Charged particles exert forces on each other. • The MORE charge (meaning more protons, and/or more electrons), the BIGGER the forces… A clump of six protons is separated from an electron by distance D. A clump of 18 protons is also separated from an electron by distance D. Which clump exerts a greater force on the electron? +++ +++ + + +++ + + ++++++ ++++ + D D Practice Problem A metal sphere has a net charge of –2.4 x 10-6 C. How many excess electrons does the sphere contain? Practice Problem A metal sphere has a net charge of –2.4 x 10-6 C. How many excess electrons does the sphere contain? GIVEN: Q = -2.4 x 10-6 C -e = -1.6 x 10-19 C #electrons = ??? Practice Problem A metal sphere has a net charge of –2.4 x 10-6 C. How many excess electrons does the sphere contain? GIVEN: Q = -2.4 x 10-6 C -e = -1.6 x 10-19 C #electrons = ??? 2.4 10 6 1 electron 15 C 1.5 10 electrons 19 1.6 10 C Law of Conservation of Charge Like other conservation laws, the law of conservation of electric charge states that the net charge (which is basically the sum of the charge on each proton and electron in a system) of an isolated system remains constant. Conductors and Insulators An electric conductor is a material, such as copper, that allows for the easy movement (conduction) of charge. In general, metals are good electric conductors because they don’t hold on to their electrons very tightly. An electric insulator is a material, such as rubber, that doesn’t allow for the easy movement of charge. Charges that Move When charges move through a solid conductor, IT IS THE NEGATIVELY CHARGED PARTICLES, THE ELECTRONS, THAT ARE FREE TO MOVE. The protons are relatively bound in space: How Might an Object Become Charged??? Charging by Friction Charging by Contact (Insulators and Conductors) Charging by Induction (Conductors Only) Charging by Friction Examples • Dragging your feet across a carpet on a dry day. • Rubbing a balloon through your hair. Charging by Friction Results in a transfer of electrons between the two objects that are rubbed together. Charging by Friction Materials at top of list have a greater affinity for electrons. When any two materials in the table are rubbed together, the one that is higher can be expected to pull electrons from the material that is lower Charging by Contact Charging by conduction is the process of giving an object a net electric charge by placing it in contact with an object that is already charged. Charging by Induction It is possible to charge a neutral conductor without contact: The Electroscope In 1748, Nollet invented one of the first electrometers, the electroscope, which can detect the presence of an electric field. Insulators Can’t Be Charged by Induction As you might expect, insulators cannot become charged by induction because charged particles are not free to move within an insulator. However, if an insulator is in the midst of an electric field, the individual molecules, while not able to move freely, may orient themselves so that there is a polarization of charge. What is Charge Polarization?? An unpolarized atom. With an external electric field, the center of electron cloud shifts to the left, or polarizes. Charge Polarization Charge Polarization Neutral objects may be a attracted to charged objects through charge polarization: Coulomb’s Law We know that charges exert forces on other charges. You are going to be given the means to calculate these forces. The force (F) between two point charges is... •Proportional to the magnitude of each charge •Inversely proportional to square of the separation between their centers (r) •Directed along the line connecting their centers F kC q1 q2 r 2 kc = 8.99 x 109 N·m2/C2 Coulomb’s Law So long as your units for charge (q) are in coulombs, and your units for distance (r) are in meters, your units for force will nicely cancel out. F kC q1 q2 Nm N 2 C r 2 2 C C 2 m Inverse Square Laws q1 q2 m1 m2 Fg G F k C 2 2 r r r/ 3 force 9F r/ 2 r 4F F F/4 2r 3r F/9