* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download CH19 Aldehydes and Ketones
Elias James Corey wikipedia , lookup
Bottromycin wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Aldol reaction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Hydroformylation wikipedia , lookup
Discodermolide wikipedia , lookup
Petasis reaction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Asymmetric induction wikipedia , lookup
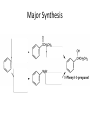
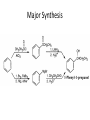
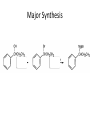
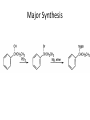
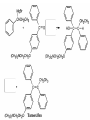
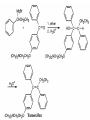
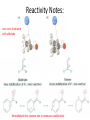
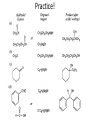
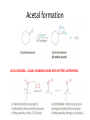
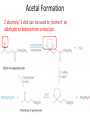
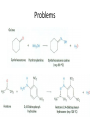
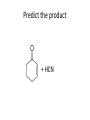
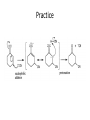
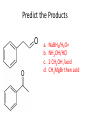
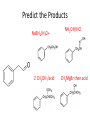
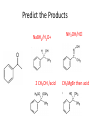
Ketones, Aldehydes CH21 PS CLASS Recall the many times we’ve synthesized these! I command thee. • Oxidation of R-OH – (Periodinane, CrO3/Na2Cr2O7) • Hydration of Alkynes (keto-enol tautomerism) – H3O+/HgSO4, BH3/H2O2,OH-, etc… • Friedel-Crafts ACYLation of Aromatics – (acid halide + AlCl3) REACTIONS OF ALDEHYDES/KETONES • Oxidation of Aldehydes • Nucleophilic Additions (overview) – Hydride (H-) and Grignard (R-) as Nucleophiles – Water addition (hydrate/diol/geminal diol formation) – Alcohol addition (acetal formation) – Amine addition (imine formation) • Conjugate Nucleophilic Addition Reactions Major Synthesis Major Synthesis Major Synthesis Major Synthesis Major Synthesis Major Synthesis Major Synthesis Oxidation of Aldehydes Where [O] = CrO3 among others. Nucleophilic Addition Rxns Slightly different mechanisms in acid or base. Neutral vs. Negatively charged Nucleophile General picture (basic): Reactivity Notes: Less steric hindrance with aldehyde Benzaldehyde less reactive due to resonance stabilization. Reduction of Ketones/Aldehydes Addition of Grignard Reagent Grignard Reagents + carbonyls Practice! COME UP WITH PAIRS, More than one answer for some. Practice! Hydrate formation/ Geminal Diols Basic vs. Acidic BASIC: Strong nucleophile attacks, as in Nu- ACIDIC: Carbonyl is converted to a stronger ELECTROPHILE as CABON BECOMES MORE POSITIVE. Problem Acetal formation ACID CATALYZED… AGAIN, CARBONYL MADE INTO BETTER ELECTROPHILE. Acetal Formation Acetal Formation Acetal Formation 2 alcohols/ 1 diol can be used to ‘protect’ an aldehyde or ketone from a reaction. Nuc. Addition of Amines to form: Imines (bisaya ka dong?) Amine must have 2 protons, RNH2 Problems Predict the Products! Problems Conjugate Nucleophilic Addition Rxns Conjugate Nucleophilic Addition Rxns THE DOUBLE BOND IS EFFECTIVELY “POLARIZED” INTO A NEGATIVE REGION THAT’S RESONANCE STABILIZED, AND A POSITIVE REGION AT THE BETA-CARBON. Predict the product + HCN Practice Predict the Products a. b. c. d. NaBH4/H3O+ NH2OH/HCl 2 CH3OH /acid CH3MgBr then acid Predict the Products NaBH4/H3O+ 2 CH3OH /acid NH2OH/HCl CH3MgBr then acid Predict the Products NaBH4/H3O+ 2 CH3OH /acid NH2OH/HCl CH3MgBr then acid