* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Energy per nucleon
Radioactive decay wikipedia , lookup
Two-dimensional nuclear magnetic resonance spectroscopy wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Nuclear fission product wikipedia , lookup
Inertial confinement fusion wikipedia , lookup
Muon-catalyzed fusion wikipedia , lookup
Isotope analysis wikipedia , lookup
Nuclear fission wikipedia , lookup
Inertial electrostatic confinement wikipedia , lookup
Isotopic labeling wikipedia , lookup
Nuclear fusion–fission hybrid wikipedia , lookup
Nuclear fusion wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Valley of stability wikipedia , lookup
Nuclear binding energy wikipedia , lookup
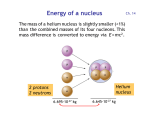
Energy of a nucleus Ch. 14 The mass of a helium nucleus is slightly smaller (<1%) than the combined masses of its four nucleons. This mass difference is converted to energy via E = mc2 . Helium nucleus 2 protons 2 neutrons 6.695·10-27 kg 6.645·10-27 kg Conversion of mass to energy 5 ·10-29 kg of mass is converted to energy when 2 protons and 2 neutrons are combined to form the helium nucleus: E = m c2 = (5·10-29 kg) · (3·108 m/s)2 = 4.5 ·10-12 J = 28 MeV Each of the four nucleons releases 28 MeV / 4 = 7 MeV 1 J = 6.24 1018 eV Energy per nucleon Energy per nucleon in MeV · 1H Principle of nuclear fusion Energy is released when combining two light nuclei into one heavier nucleus. 4He Ni Nucleon Number A Fe and Ni have are the most stable nuclei (lowest energy per nucleon). Fusion vs. fission • Energy is released either by combining two small nuclei (fusion) or by splitting a large nucleus into two pieces (fission). Pu • The energy is released as radiation and as kinetic energy. Both eventually turn into heat (the fireball from a nuclear bomb and the steam generated in a nuclear reactor). Plutonium Pu Fe Ni Nucleon Number A Fusion vs. fission in bombs, reactors • Fusion powers hydrogen bombs. Fission powers atomic bombs. • Fusion has not yet been tamed for peaceful purposes. Fission generates energy in nuclear reactors. Fusion in stars • Stars convert hydrogen to helium and heavier elements. When Fe and Ni are reached, fusion stops. The star has burnt its nuclear fuel and collapses under its own gravity. • In massive stars, this collapse releases a huge amount of gravitational energy that leads to a supernova.The outer 90% of the star is ejected, and the center becomes either a black hole (> 3 solar masses) or a neutron star (between 1.4 and 3 solar masses), where the atoms collapse into a single huge nucleus. Lighter stars become white dwarfs . • All elements heavier than iron/nickel are created during a supernova explosion, which has enough thermal energy to form nuclei with higher energy per nucleon. Stable nuclei Red dots = stable nuclei. The gray region contains unstable nuclei, created in the laboratory. Stable nuclei have about equal neutron and proton numbers N and Z (dashed). At high Z, there are more neutrons than protons, because protons are charged and repel each other. Radioactive decay If the ratio of protons to neutrons gets too far off-balance, a nucleus will spontaneously transform itself into another nucleus with a better ratio by emitting , , particles. particle = 2p 2n = He nucleus particle = electron particle = photon Marie Curie, Nobel prizes in physics, chemistry Isotopes Isotopes are different versions of the same element (same Z). They have the same number of electrons and protons , but a different neutron number N. Their chemical behavior is the same, since that is determined by the electron number (= Z). Stable isotopes are shown as red dots.The gray region contains unstable isotopes which are radioactive. Different isotopes of the same element Isotopes of hydrogen Hydrogen One proton Deuterium One proton one neutron Tritium One proton two neutrons These three isotopes play a central role in various fusion reactions. Isotopes of carbon • Carbon has 6 protons and 6 electrons (Z=6). Its outer shell contains 4 electrons, which determine the chemical properties of carbon. • The most common isotope of carbon has 6 neutrons, 12 nucleons. It is commonly labeled 12C (“C twelve”). • 14C is another isotope of carbon containing 8 neutrons, 14 nucleons. • 14C is unstable and decays radioactively. Half-life The decay of 14C is exponential (Lect. 4, Slides 5,6). After 6000 years, half of the 14C has decayed (= half-life). After another 6000 years, one loses another half, and so on every 6000 years. Carbon-dating question The 14C/12C ratio in a fossil bone is found to be ⅛ of the ratio in a living animal. What is the approximate age of the fossil ? A. 6 000 years B. 18 000 years C. 32 000 years D. 48 000 years Since the ratio has been reduced by a factor of ⅛ = ½½½ = (½)3, three half-lives have passed, i.e. 3 · 6000 years = 18 000 years Radioactive dating • Radioactive 14C is created continuously by cosmic rays (next slide). • 14C oxidizes to CO2 and is converted by plants into organic matter. Particularly durable are wood and charcoal generated from wood. • Animals and humans eat plants and incorporate 14C into the bones. • Decaying 14C is replenished as long as plants and animals are alive. • Once a plant or animal dies, its 14C content decreases and thereby starts the clock for radiocarbon dating. • By measuring the 14C/12C ratio of a sample from an archaeological site one can determine its age. (Willard Libby, 1969 Nobel Prize) • This can be done up to an age of about 60 000 years, when the 14C concentration has been reduced by a factor of (½)10 = 1/1024 . Production of carbon 14C A cosmic ray proton shatters the nucleus of an atom in the upper atmosphere, creating neutrons n plus other debris. A 14N nucleus absorbs a neutron and emits a proton, becoming 14C . Concentration of 14C • A balance between the production and decay rates determines the equilibrium ratio: 14 C 12 1.3 10 12 C • Such an extremely low ratio of one part in a trillion requires a very sensitive detector which can detect single 14C atoms. • It helps to have a large number of C atoms from a macroscopic sample (compare Avogadro’s number, 1024 ). Geological dating • For older specimens one uses isotopes with longer half-life, for example 235U (uranium). Its half-life is 0.7 billion years. • The oldest rocks on Earth have been dated this way. These are 4.4 billion years old. • A focused ion beam removes a small amount of material from several spots on one of the tiny red zircon crystals.