* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Fatty acid metabolism wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Biosynthesis wikipedia , lookup
Phosphorylation wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Citric acid cycle wikipedia , lookup
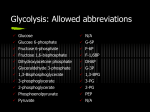
FUNdamentals 1 09/03/08 (Dr. Pritchard) 11:00 – 12:00 Introduction: Today, I am going to speak about the opposite process of what Dr. Miller talked about, which is making new glucose (gluconeogenesis). It is what it sounds like, the synthesis of new glucose. Slide 1 - Gluconeogenesis People require a lot of glucose (about 160 grams a day). That is a lot of sugar. It is about a half full cup and everyone needs this much. Why does the brain need so much sugar? The answer is that the brain is filled will billions of neurons that have to maintain electrical potentials and it takes a lot of ATP to do that. o The brain uses glucose to make the ATP and maintain these electrical potentials in the neurons. We have a little glucose in our blood and other body fluids. If you haven’t been exercising, you have a fair amount of glucose in your muscle and liver (180-200g) Bottom line: if you don’t get enough glucose in your diet, then you will have to make your own, and that process is called gluconeogenesis IMPORTANT: 90% of gluconeogenesis occurs in the liver and kidneys Slide 2 - Historical Perspective It turns out that seven of the same enzymes used in gluconeogenesis are also used in glycolysis. The reactions will be catalyzed in the reverse direction though. Eduard Buchner was a biochemist (Buchner funnel) and he had a microbiologist brother and they decided they were going to make a lot of money selling yeast extract because it was believed to be healthy (and it is because it is filled with Bvitamins). The problem was how do you package it? They didn’t want to heat it up, sterilize it and package it because they thought they would inactivate it. Storing processes were very limited during this time. A common way to preserve things in those days was to salt it down. This doesn’t work with something that is liquid because then you don’t have any way to remove the salt. The other way things were often preserved is they were dumped in with a lot of sugar (sucrose). o High osmolarity of the sugar keeps bacteria and mold from growing on it. They added sugar to the yeast and the whole thing began to smell like alcohol because of anaerobic glycolysis. This was interesting to them because up until then people believed that fermentation could only occur with living organisms. Slide 3 - Historical Perspective (cont’d) Other people after them discovered that this yeast extract could be separated into two fractions. One fraction would go through a porous filter, cozymase, and another fraction would not go through the filter and that was called zymase. Also found that phosphate was required and became linked to sugar during the process. Other people found the same enzymes in muscle. Remember, this study was done in yeast. o Virtually the same enzymes with a few exceptions. Muscle does not make alcohol. In the absence of O2, the end product of glycolysis is lactic acid Slide 4 – Enzyme differences between glycolysis and gluconeogenesis If you read this diagram downwards it is glycolysis. If you read this diagram upwards it is gluconeogenesis. You should learn this table! Know the differences. o Some are unique to glycolysis, some are unique to gluconeogenesis. Seven are common. Slide 5 - First Reaction of Gluconeogenesis First step: conversion of pyruvate to oxaloacetate This is the overall reaction, more detail will be presented in the next slide Overall, you have pyruvate (a simple, 3 carbon, keto-acid) and bicarbonate (buffer in the blood…made by mixing carbon dioxide and water) This process requires ATP and you make oxaloacetate. You’ve added a CO2 to pyruvate Slide 6 – Biotin & Avidin Reactions that add carbon dioxide almost always use a cofactor called Biotin, which is often considered a vitamin. You rarely find deficiencies of Biotin, unless people are on a really weird diet. Even the bacteria in our guts make Biotin. Someone’s diet must be really messed up to have a lack of Biotin (e.g. eating lots of raw eggs) The egg white contains a protein called Avidin, which tightly binds Biotin. It is almost a covalent binding, but not quite. VERY TIGHT binding. o It makes the Biotin unavailable. o And in this particular case people can actually get a Biotin deficiency. Note the structure of Biotin on slide o 2 rings (one with a sulfur, one with two nitrogens) o The carbon dioxide ends up being attached here (bottom right N-H bond in the green shading) o There is also a “flexible leash”…in the enzyme, Biotin is always covalently bound to a imide bond, to a lysine…basically you have the Biotin on a long leash and the way that this works is that it will pick up carbon dioxide and then swivel a great distance to another part of the enzyme for the next step, the transfer of the carbon dioxide . Slide 7 – Biotin & Oxaloacetate Involvement This reaction is in several steps, but it is catalyzed by the same enzyme First of all, you end up making carbonyl phosphate (a carbon dioxide linked to a phosphate) so what you are doing is activating carbon dioxide Then, the carbon dioxide is transferred to the nitrogen of Biotin and you get a new compound which reacts with pyruvate that has been deprotonated (pyruvate is at the top right hand corner of slide) to make oxaloacetate Step 1: converting pyruvate to oxaloacetate Slide 8 - Oxaloacetate This occurs in the mitochondria. The problem is there is no transporter molecule that can move oxaloacetate from the inside of mitochondria to the outside. o What happens is oxaloacetate is reduced (with NADH) to malate Malate CAN be transported out because there is a transporter for it. Once it is out, it is reoxidize to oxaloacetate again. This is called a shuttle. Slide 9 - The PEP carboxykinase reaction. The second step in gluconeogenesis is converting oxaloacetate to PEP. Note GTP instead of ATP in slide (for regulatory reasons) Net effect is you make PEP CO2 is removed even though you go through all the trouble w/ Biotin to put it on The reason you do this is because PEP is a very high-energy compound. What you’ve done is you’re essentially using two ATPs because one ATP is not enough to convert In the first step you are adding the CO2 and using an ATP In the second step you are using GTP (equivalent to an ATP) and in effect, you are using two high-energy molecules to make this because one simply does not have enough energy. o The cleaving off of the CO2 is energetically favorable & helps drive the reaction forward This is a common type of series of reactions seen in Biochemistry. You are pushing a reaction uphill that would normally be energetically unfavorable, but you do it by coupling it to the hydrolysis of two high-energy bonds of ATP or ATP like molecules. Slide 10 – PEP formation “This is the same thing.” Slide 11 – Glycolysis Pathway Reading downward = glycolysis; upward = gluconeogenesis The 4 stars on the reaction are reactions that are unique to gluconeogenesis, but most of the reactions here (with double arrows) are common with both glycolysis and gluconeogenesis Slide 12 - Enolase Reaction The third reaction is the conversion of PEP to 2-phosphoglycerate (2-PG) This is basically a hydration reaction where the water adds to the double bond Slide 13 - The Phosphoglycerate Mutase Reaction Next reaction, you move the phosphate from the 2 3 position and the enzyme that does that is phosphoglycerate mutase Slide 14 - Isomerase, Kinase and Mutase A mutase is an enzyme that catalyzes the transfer of a functional group (such as phosphates, sulfates, acetyl groups, etc ) from one position to another. This could be a good exam question. Most of the time when you think of a kinase you think of an enzyme that catalyzes the addition of a phosphate from an ATP to something. But it can also catalyze the reverse reaction. You can get a phosphate from some other molecule adding to ADP that will make an ATP. If the ATP is involved in phosphate transfer (either getting or removing) that is called a kinase Almost always where we are talking about a carbohydrate enzyme and it has “isomerase” in the title, you know it is talking about converting a ketose to an aldose or vice versa Remember these terms because some of the enzymes we will encounter will have these terms Slide 15 – (1,3-BPG) (3-PG) The next reaction of gluconeogenesis We now have the 3-PG and what happens is with ATP you add another phosphate to the carboxylic acid group. This molecule (far left molecule: 1,3 – Bisphosphoglycerate) is a mixed anhydride, but it is a carboxylic acid activated with phosphate. Slide 16 - The glyceraldehyde-3-phosphate dehydrogenase reaction You’ve seen this enzyme before in glycolysis going the other direction In gluconeogenesis, it catalyzes the conversion of 1,3-BPG G-3-P This NADH has reduced this carboxylic acid group to an aldehyde. Slide 17 – DHAP G-3-P In glycolysis, triose phosphate isomerase converts G-3-P DHAP If DHAP didn’t have a phosphate group on it, then it would be dihyrdoxyacetone (DHA) and it stains your skin yellow and is used in self tanning lotion In this slide, all this is showing is that these molecules can be readily converted This particular enzyme is considered a perfect enzyme. It is almost as if every collision of the enzyme with G-3-P results in a conversion. It is a very efficient enzyme. Slide 18 – Glycolysis Pathway Where are we on this pathway? “This point” (at the position of G-3-P/DHAP transition). o Remember in glycolysis, these are the two molecules that are produced by the breakdown of fructose 1,6-bisphosphate In gluconeogenesis these molecules are joined to make fructose 1,6-bisphosphate. The same enzyme does the job: aldolase Slide 19 - Aldolase When you join these two small molecules (one a ketone and one an aldehyde) that is called an aldol condensation. In glycolysis, it is called an aldol cleavage when you break it down Remember, you see all these reactions in pathways. You may wonder why you don’t go from glucose to pyruvate. Why all the steps? The problem is you are trying to set the molecule up so that it will be in a form where you can join two molecules together, or cleave them, depending on which direction you are going. The problem in Biochemistry is you have to stay at body pH…it is not like Organic Chemistry when you can do things that can’t be done in a human cell. (Dr. Pritchard elaborated a little bit on this concept, but just stressed that this pathway occurs at body temperature and you are limited in your reactions) Slide 20 – Aldol Condensation Shows you what I said earlier. By setting these things up the right way you can either cleave them (glycolysis) or join them (gluconeogenesis) Slide 21 – Diagram: Dihydroxyacetone phosphate & G-3-P People have done X-ray crystallography of aldolase and they can tell you exactly how the molecule is oriented in space. Note the two molecules in the slide. Slide 22 – Glycolysis Pathway Now we are at this point. We have made fructose 1,6-bisphosphate. Note the star, which means the next reaction is unique to gluconeogenesis Slide 23 – Fructose 1,6-bisphosphate Look at the structure for a second o The 6th position with the phosphate on it o The 1st position with the phosphate on it What happens in this reaction is that the Fructose 1,6-bisphosphotase cleaves off a phosphate. Remember from glycolysis that the opposite reaction requires an ATP; there is no ATP involved here, you don’t generate an ATP Slide 24 – Diagram: Glucose-6-phosphate Fructose-6-phosphate Fructose 6-phosphate is converted to Glucose 6-phosphate and the enzyme is Phosphoglucose isomerase. Remember, when you see isomerase in an enzyme name it means you are converting an aldose to a ketose or vice versa Here you are converting a ketose to an aldose Slide 25 – Transferring Glucose-6-phosphate into the cytoplasm When glucose is taken up by cells, almost all cells will phosphorylate it using an ATP and hexokinase. Once this happens the glucose cannot get out. It has a negative charge and is trapped within the cell. Slide 26 - Glucose-6-phosphatase Most cells don’t have an enzyme that will take the phosphate off the glucose again, except the liver and the kidneys (where gluconeogenesis occurs) The way this works is that G-6-P gets transported into the endoplasmic reticulum and then a membrane bound enzyme (glucose 6-phosphatase) cleaves off the phosphate, the glucose gets into the lumen of the ER, gets packaged into vesicles that move to the plasma membrane of the cell where they fuse and the glucose is released into the blood stream. This is a good exam question: Where does this reaction take place?” Slide 27 - The Cori Cycle Husband and wife biochemist team that won The Nobel Prize They noticed that when animals do vigorous exercise you don’t have enough oxygen to do it all aerobically, so you end up doing anaerobic glycolysis and that has an effect of producing lactic acid. Remember why you are making this lactic acid. When you do glycolysis you convert your NAD to NADH. You rapidly start running out of NAD and they way that it is normally regenerated is the electron transport chain in the mitochondria and that requires oxygen (oxidative phosphorylation). If you can’t do that because you don’t get enough oxygen, then you have real problems with not enough NAD. They way you regenerate it is you turn it into lactic acid and that regenerates your NAD as depicted in the slide. What these people found out is that this lactic acid from muscles gets into the bloodstream, travels to the liver where the lactic acid is convereted back to pyruvate and then that pyruvate (starting material for gluconeogenesis) goes on to make more glucose and that glucose gets put into the bloodstream where it is carried to the muscle and broken down again to get lactate…and so on and so on o This is the so-called “Cori Cycle” Slide 28 - Regulation of Gluconeogenesis At one particular stage “substrate level control” is present where: a lot of G6P builds up in the liver or kidney, then it activates the glucose-6-phosphatase (enzyme in the ER membrane) that cleaves the phosphate off to get glucose. Glycolysis and gluconeogenesis are reciprocally regulated Something that will stimulate glucose breakdown (glycolysis) almost always will inhibit gluconeogenesis or vice versa You don’t want both going on at the same time because you will waste ATP, sometimes this occurs in newborn babies to keep them warm Slide 29 – Fructose 2,6-bisphosphate This molecule looks similar to a molecule seen before (Fructose 1,6bisphosphate…the intermediate in gluconeogenesis and glycolysis), but this molecule has a phosphate in the second position, not the first This is involved in the pathway as a regulatory molecule (only small amounts are made, but has a powerful effect) The small portions of this molecule activates phosphofructokinase (the key enzyme in glycolysis), in other words, if you want to breakdown glucose fast, you need a little of this regulatory molecule in your bloodstream At the same time, it inhibits fructose 1,6-bisphosphate which is a key enzyme in gluconeogenesis o If you’re breaking down glucose, you don’t want to waste energy and ATP to make more of it The enzyme that makes this and breaks it down are joined together o Same bifunctional enzyme Slide 30 - Fructose-2,6-bisphosphate The breakdown fructose 2,6-bisphosphate requires the cleaving off of a phosphate and that takes a special phosphatase To make fructose 2,6-bisphosphate you cleave an ATP to add a phosphate and that takes a special kinase Slide 31 - 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase People have observed the crystal structure of these thing and discovered there are two major domains and a minor one o Phosphatase domain: involved in breaking down the regulatory molecule o Kinase domain: involved in adding the phosphate o Regulatory region Slide 32 - 6-phosphofructo-2-kinase/fructose-2,6-bisphosphatase When blood-glucose levels get low a hormone called glucagon is produced and gets into the blood The net effect of this is to cause increased phosphorylation of a lot of molecules Glucagon tends to have the opposite effect of insulin Insulin triggers an increase in the phosphatase activity (takes phosphate off) When you have low glucose, it increases the phosphorylation and the net effect is it inactivates the kinase (makes the regulatory molecule) and activates the phosphatase (breaks the regulatory molecule down) The idea is if you have low glucose, the last thing you want to do is break down that little bit of glucose you have by glycolysis…you want to shut it down You want to make glucose as fast as you can by gluconeogenesis You can memorize these concepts, or just think about them. They make sense! o Low glucose = no breakdown of it, make more of it o High glucose = use it, break it down You can rationalize these regulatory steps Slide 33 – Diagram: Regulating Gluconeogenesis and Glycolysis You can rationalize almost all of these concepts on this slide Why carry out glycolysis in the first place? To break down glucose & make ATP o If you have lots of ATP, there is no need for glycolysis Note the plus and minus symbols on the slide o Minus = negative regulator, or inhibitor of the enzyme Example: this enzyme is Phosphofructokinase is a key enzyme in glycolysis, so lots of ATP will inhibit this enzyme that is involved in making more ATP If you have lots of ATP, you should start making more glucose by gluconeogenesis; you can afford it, you have enough ATP o Plus = activates enzyme Low ATP values means AMP levels are high AMP positively activates this enzyme AMP will negatively affect the other enzyme (Fructose – 1,6-bisphosphatase) Again, you can rationalize these concepts. Slide 34 - Substrates and Non-substrates for Gluconeogenesis: What substrates do we use for making glucose o Pyruvate o Lactate – you can always convert lactate to pyruvate o TCA cycle intermediates o Most amino acids In the breakdown of amino acids, you will see that many of them are converted to keto-acids (e.g. alpha-ketoglutarate) that are TCA cycle intermediates Notice what is not a substrate: o Acetyl-CoA o Fatty acids o Lysine & Leucine (all other amino acids can be) Nice exam question Basic idea here is something that you will likely get on your board exams in one form or another. The idea is that animals are unable to convert fat into glucose. That is the key point. Loads of fat can provide your energy needs for months. That is not good enough if you are starving because you can’t convert that fat into glucose. Your brain and kidneys need glucose. You need this glucose and you have to make it if you are starving and you need to use gluconeogenesis for that If you starve you are going to lose protein in your muscles and that protein is broken down to amino acids, which in turn are broken down into TCA cycle intermediates Eventually you get oxaloacetate, which is used for making sugar Starvation is a terrible way to diet! You looks your muscles. Low carb diets still have a lot of protein and you use that protein to make sugar because your brain needs it! Slide 35 – Diagram: Citric Acid Cycle Why is it that you can’t convert fat to glucose? o All you need to do is somehow get it converted to oxaloacetate (on pathway to make more glucose), then you could make sugar out of it When you break down fat, you get Acetyl-CoA (cofactor that carries acetyl group that has two carbons in it) In the Citric Acid Cycle you split off two CO2 o No matter how much Acetyl-CoA you put in, you are not going to get any more oxaloacetate o You have loads of energy (in ATP form), but no more oxaloacetate, therefore no more sugar However, breaking down proteins into amino acids can be converted to alphaketoglutarate; this works and makes more oxaloacetate Amino acids/Proteins can be used to make sugar Main point: Acetyl-CoA coming from fat is not good enough to make sugar for an animal Slide 36 - Plants and bacteria can make glucose from acetate. However, plants can do it They use the Glyoxylate Cycle Notice there is no carbon dioxide split off Two types of enzymes here that animals don’t have o Isocitrate lyase Cleaves the isocitrate (instead of splitting a CO2) Cleaves into molecules called glyoxylate and succinate (in Citric Acid Cycle) o Malate synthase Can add an Acteyl-CoA to glyoxylate to make malate (in Citric Acid Cycle) There is a shunt that avoids the two steps that split off the carbon dioxide This just reinforces the idea that animals cant turn their fat into glucose Slide 37 – Comparison of the TCA Cycle and Glyoxylate Cycle Comparison of the two cycles here Slide 38 – Pyruvate Glucose Another illustration of gluconeogenesis Slide 39 – Enzymatic differences in glycolysis and gluconeogenesis Remember this table! Slide 40 – Blank Slide