* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Microbial Metabolism - ASAB-NUST
Fatty acid synthesis wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Nitrogen cycle wikipedia , lookup
Photosynthesis wikipedia , lookup
Biochemistry wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Metalloprotein wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Citric acid cycle wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Electron transport chain wikipedia , lookup
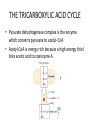
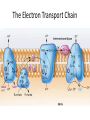
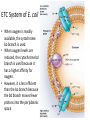
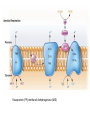
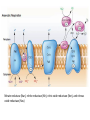
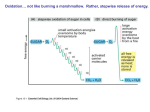
LECTURE 11: Microbial Metabolism Microbiology and Virology; 3 Credit hours Atta-ur-Rahman School of Applied Biosciences (ASAB) National University of Sciences and Technology (NUST) THE TRICARBOXYLIC ACID CYCLE • Pyruvate dehydrogenase complex is the enzyme which converts pyruvate to acetyl-CoA • Acetyl-CoA is energy rich because a high energy thiol links acetic acid to coenzyme A. The Tri-carboxylic Acid Cycle • TCA cycle enzymes are widely distributed among microorganisms. • In procaryotes, they are located in the cytoplasmic matrix. • In eucaryotes they are found in the mitochondrial matrix. • The complete cycle appears to be functional in many aerobic bacteria, free-living protists, and fungi. The Electron Transport Chain • Electron transport chain is composed of a series of electron carriers that operate together to transfer electrons from donors, like NADH and FADH2, to acceptors, such as O • The electrons flow from carriers with more negative reduction potentials to those with more positive potentials and eventually combine with O2 and H to form water. • The difference in reduction potentials between O2 and NADH is large, about 1.14 volts, which makes possible the release of a great deal of energy The Electron Transport Chain The Electron Transport Chain ATP Synthase ETC System of E. coli • When oxygen is readily available, the cytochrome bo branch is used. • When oxygen levels are reduced, the cytochrome bd branch is used because it has a higher affinity for oxygen. • However, it is less efficient than the bo branch because the bd branch moves fewer protons into the periplasmic space flavoprotein (FP),methanol dehydrogenase (MD) Nitrate reductase (Nar), nitrite reductase (Nir), nitric oxide reductase (Nor), and nitrous oxide reductase (Nos) • Bacteria such as Desulfovibrio are another example. They donate eight electrons to sulfate, reducing it to sulfide (S2 or H2S). • The anaerobic reduction of nitrate makes it unavailable to the cell for assimilation or uptake. Therefore this process is called dissimilatory nitrate reduction. • P. denitrificans avoid the toxic effects of nitrite by reducing it to nitrogen gas, a process known as de-nitrification.