* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Decarboxylation Reactions Major concepts Decarboxylation
Survey
Document related concepts
Deoxyribozyme wikipedia , lookup
Microbial metabolism wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Biochemistry wikipedia , lookup
Photosynthesis wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Metalloprotein wikipedia , lookup
Biosynthesis wikipedia , lookup
Transcript
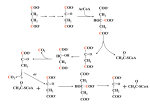
Decarboxylation Reactions Major concepts Decarboxylation reactions result when a carboxylic acid (or carboxylate) decompose to give carbon dioxide. They can occur under acidic or basic conditions. Decarboxylation reactions are not redox reactions, but are often linked to oxidation reactions. When a carboxylate is “Beta” to a carbonyl, spontaneous decarboxylation can result and requires no cofactor. When a carboxylate is “Alpha” to a carbonyl, decarboxylation requries a cofactor called Thiamine pyrophosphate (TPP) to stabilize the intermediates in the reaction (electron sink.) When a carboxylate is “alpha” to an amine (i.e. “amino acid”), decarboxylation requires pyridoxal phosphate (PLP) as a cofactor to stabilize the intermediates in the reaction (electron sink.) Vocabulary Decarboxylation Beta decarboxylation vs. alpha decarboxylation vs decarboxylation of amino acids Electron sink Students should be able to: Draw a mechanism for Beta-decarboxylation under basic conditions Determine when a decarboxylation requires a cofactor and when it does not. Recognize an oxidative decarboxylation, and explain why the oxidation was linked to decarboxylation. Daily Problems 1. Provide a non-enzymatic mechanism for this decarboxylation: O O heat O- H2O O + CO2 2. Label each reaction as “oxidation”, “decarboxylation”, “oxidative decarboxylation” or “none of these.” 3. Refer to the following figure to answer the questions below: O NH3+ HO H HO OPi + HO -O N H O Phase 1 HO N OPi -O HO O HO NH CO2 Phase 2: HO N OPi HO H+ HO NH Phase 3 HO N OPi Phase 4 H HO HO NH A. B. C. D. What is the name of the coenzyme in this series of reactions? How would you describe the reaction in Phase 1? Draw mechanism arrows for Phases 2 and 3. If Phase 4 is a hydrolysis, draw the products of this reaction. 4. In the process below, pyruvic acid is converted to acetaldehyde. In the mechanism provided, all intermediates are given. Fill in the missing mechanism arrows for all four steps. (Note: An enzyme provides the proton necessary for step 3. O O S O- O- - C O R + S N C R O R N R O - O - O H S H S + H+ C S C R R C N R N R R N R 5. During gluconeogenesis, oxaloacetate is decarboxylated. a. Which carboxy group will be decarboxylated without a coenzyme? b. Why will the other carboxy group not be decarboxylated? c. Besides carbon dioxide, what is the other product of the reaction? O - decarboxylation O OO O CO2 + 6. During the citric acid cycle, isocitrate is converted into -ketoglutarate through a two step process. a. Label each step with the type of reaction. b. Which coenzyme, if any, is necessary for each step? Why is it necessary? c. Refer to the structures of the coenzymes to provide a mechanism for each step of the reaction. a. If the two steps were switched, this process wouldn’t work. Why? OH - O - O O O - O O- O O O - O + H+ O - O - O O O H O O- O 7. The following reaction is part of the process by which tryptophan is converted to serotonin, a neurotransmitter. a. Label each step with the type of reaction. b. Which coenzyme, if any, is necessary for the second step of this reaction? Why is it necessary? O O H2N CH C OH H2N CH C OH H2N CH2 CH2 CH2 CH2 OH HN tryptophan HN HN serotonin 8. Pyruvate dehydrogenase is an enzyme that converts pyruvate to acetyl CoA through a series of chemical reactions. Although this is a simplified scheme, the pathway generally follows this path: O O Coenzyme A and another cofactor O cofactor H O SCoA O- a. Label each step with the type of reaction. b. Which coenzyme is necessary for each step of this reaction? Why is it necessary? 9. As part of the citric acid cycle, a-ketoglutarate must be decarboxylated. a. What cofactor is necessary? What is its purpose? b. Fill in arrows in the figure below to complete the mechanism. O S C R O-OOC N + R O O - O - O - O S C S C R -OOC N R -OOC R N R H+ O - O -OOC H H S -OOC + C R N S C R R N R 10. The following figure contains the citric acid cycle. A. Label one reaction as an “aldol reaction,” one reaction as an “electrophilic addition,” and one reaction as a “hydrolysis reaction.” B. Label all reactions in which CO2 is produced. If a cofactor is required, give the cofactor. C. Label all reactions that are oxidations. O O H3C O- * O CoA H3C S * C COO- H2C COO- O HO H2C COO- C COO- H2C COO- * H C HO COO- H2C H2C COO- HC COO- C H COO- COO- HO * * H COOC H2C COO- C -OOC CH2 H O * H2C COO- H2C CH2 O C COO- CH2 O- * O C * SCoA C COO-