* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Organic Molecules
Protein (nutrient) wikipedia , lookup
Protein moonlighting wikipedia , lookup
Circular dichroism wikipedia , lookup
Protein structure prediction wikipedia , lookup
List of types of proteins wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Nucleic acid analogue wikipedia , lookup
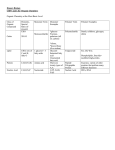
Biochemistry Organic Molecules Target #20- I can differentiate between inorganic and organic molecules Inorganic molecules constitutes non- living matter that play important roles in living things Ex: NaCl Organic molecules constitutes living matter Always contains carbon and hydrogen Accounts for the formation of a variety of organic molecules Macromolecules Known as the “molecules of life” Made most of elements like carbon and hydrogen Monomer: a simple organic molecule that exists individually A basic unit of a macromolecule Polymer: many monomers linked together Linked through a dehydration reaction Removal of a water molecule Target #21- I can differentiate between a monomer and a polymer Each macromolecule has a corresponding monomer and polymer Types of macromolecules Proteins lipids Carbohydrates Nucleic acids Target #22- I can list the 4 macromolecules Target #23- I can describe the function of a carbohydrate Carbohydrates Function for quick fuel and short-term energy storage Play a structural role in woody plants, bacteria, and animals like insects Are involved in cell-to-cell recognition Characterized by the presence of the atomic grouping H-C-OH The monomer of carbohydrates is a monosaccharide Ex: glucose Glucose is used as an immediate source of energy in both plants and animals Ex: fructose Found in fruits Disaccharide: contains two monosaccharides that have joined together Ex: The sucrose & lactose polymer of a carbohydrate is a polysaccharide Target #24- I can identify the monomer and polymer of carbohydrates Target #25- I can identify the 3 polysaccharide molecules of carbohydrates Starch is a storage molecule in plants Glycogen is a storage molecule in animals Found in the liver Cellulose cell walls is found in plant Makes plants hard to digest Biochemistry Proteins Target #26- I can describe the function of a protein Perform many functions Provides support structures Keratin hair and nails Collagen ligaments, tendons, and skin Controls metabolism Some proteins are enzymes Enzymes speed chemical reactions Hormone production Muscle function Transportation of molecules in the blood Cellular transport of molecules The monomer of a protein is an amino acid The polymer of a protein is a polypeptide Made up of 2 or more amino acids Amino acids are bonded via a polypeptide bond Occurs between the oxygen, carbon, nitrogen, and hydrogen atoms Polypeptides vary in shape depending on the composition Target #27- I can identify the monomer & polymer of a protein The shape of a protein is important to its function When proteins are exposed to extremes in heat and pH, they denature An irreversible change in shape Ex: heating an egg white cases it to coagulate Occurs because the normal bonding between the polypeptides has been disturbed Once a protein loses it’s shape, it can no longer perform its function Target #28- I can explain why the shape of a protein is important to its function Bellwork In what foods or organisms do we find lipids? Biochemistry Lipids Target #29- I can describe the function a lipid Fats and oils function as energy storage molecules Phospholipids form a membrane around the cell Basis for steroids, like estrogen and testosterone Diverse in structure and function Common characteristic do not dissolve in water Aka: hydrophobic Do not have a monomer or a polymer Target #30- I can describe the structure and function of fats & oils Fats & Oils & butter oil & soybean oil energy storage Insulates against heat loss Forms a protective cushion around major organs Oils are liquid at room temperature Corn Function Long-term Most familiar lipids Fats are solid at room temperature Lard Structure Made of a glycerol & 3 fatty acid chains Called triglycerides Target #31- I can differentiate between saturated and unsaturated fatty acids Fatty acid A hydrocarbon chain that ends with the acidic group COOH Either saturated or unsaturated Saturated fatty acids have no double covalent bonds between carbon atoms The carbon chain is saturated with all the hydrogens it can hold Account for the solid nature of fats, like butter, at room temperature Unsaturated fatty acids have double bonds between carbon atoms wherever the number of hydrogens is less than two per carbon atom Accounts for the liquid nature of vegetable oils at room temperature Phospholipids Lipids that contain a phosphate group The polar phosphate bonds to two fatty acid groups The phosphate group is polar Hydrophilic Fatty polar acid tails are non- Hydrophobic Primary components of cellular membranes Form a bilayer where the heads are on the outside, and the tails are on the inside Target #32- I can explain the structure of a phospholipid Nucleic Acids Target #33- I can describe the monomer of a nucleic acid The monomer of a Nucleic Acid is a nucleotide Nucleotides consist of a phosphate, a pentose sugar, and a nitrogencontaining base Five nitrogen containing bases adenine Guaninie Thymine Cytosine Uracil The polymer of a nucleic acid is DNA (deoxyribonucleic acid) and RNA (ribonucleic acid) DNA is made of the monomers adenine, guanine, cytosine, and thymine RNA is made of the monomers adenine, guanine, cytosine, and uracil Target #34- I can identify the polymers of nucleic acids Target #35- I can explain how nucleotides form a polymer The nucleotides form a linear molecule called a strand Alternating phosphates and sugars create a back bone In DNA, two strands twist together to form a double helix Held together by hydrogen bonds The bases bond together to make “rungs” like in a ladder In RNA, a single strand forms Used to complement DNA