* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 29
Fallout shelter wikipedia , lookup
Nuclear fission product wikipedia , lookup
Gamma spectroscopy wikipedia , lookup
Ionizing radiation wikipedia , lookup
Nuclear fission wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Technetium-99m wikipedia , lookup
Two-dimensional nuclear magnetic resonance spectroscopy wikipedia , lookup
Nuclear fusion wikipedia , lookup
Nuclear transmutation wikipedia , lookup
Nuclear binding energy wikipedia , lookup
Radioactive decay wikipedia , lookup
Valley of stability wikipedia , lookup
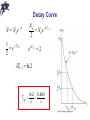
Chapter 29 Nuclear Physics Properties of Nuclei • All nuclei are composed of protons and neutrons (exception: ordinary hydrogen) • Nucleon is a generic term used to refer to either a proton or a neutron • The atomic number, Z, equals the number of protons in the nucleus • The neutron number, N, is the number of neutrons in the nucleus • The mass number (not the same as the mass), A, is the number of nucleons in the nucleus: A = Z + N • Symbol: A Z Properties of Nuclei X • X is the chemical symbol of the element • Example: 27 13 Al • Mass number is 27; atomic number is 13; contains 13 protons and 14 = 27 – 13 neutrons • The Z may be omitted since the element can be used to determine Z • The nuclei of all atoms of a particular element must contain the same number of protons Properties of Nuclei • The nuclei may contain varying numbers of neutrons • Isotopes of an element have the same Z but differing N and A values. For example: 11 6 C 12 6 C 13 6 C 14 6 C • The proton has a single positive charge, +e (e = 1.602 177 33 x 10-19 C) • The electron has a single negative charge, -e • The neutron has no charge, which makes it difficult to detect Mass • It is convenient to express masses using unified mass units, u, based on definition that the mass of one atom of C-12 is exactly 12 u: 1 u = 1.660 559 x 10-27 kg Masses Particle kg u 1.6726 x 10-27 1.007276 Neutron 1.6750 x 10-27 1.008665 Electron 9.109 x 10-31 5.486x10- Proton 4 The Size of the Nucleus • From scattering experiments, Rutherford found an expression for how close an alpha particle moving toward the nucleus can come before being turned around by the Coulomb force • The KE of the particle must be completely converted to PE 2 2 mv q1q2 (2e)( Ze) ke ke 2 r d • d gives an upper limit for the size of the nucleus (e.g., for gold, d = 3.2 x 10-14 m and for silver, d = 2 x 10-14 m) 4ke Ze d mv2 The Size of the Nucleus • Such small lengths are often expressed in femtometers where 1 fm = 10-15 m Also called a fermi • Since the time of Rutherford, many other experiments have concluded that most nuclei are approximately spherical with the average radius of 1 3 r ro A • ro = 1.2 x 10-15 m Enrico Fermi 1901 – 1954 Chapter 29 Problem 7 (a) Find the speed an alpha particle requires to come within 3.2 × 10−14 m of a gold nucleus. (b) Find the energy of the alpha particle in MeV. Nuclear Stability • There are very large repulsive electrostatic forces between protons • These forces should cause the nucleus to fly apart • The nuclei are stable because of the presence of another, short-range force, called the nuclear force • This is an attractive force that acts between all nuclear particles • The nuclear attractive force is stronger than the Coulomb repulsive force at the short ranges within the nucleus Nuclear Stability • Light nuclei are most stable if N=Z • Heavy nuclei are most stable when N > Z • As the number of protons increase, the Coulomb force increases and so more nucleons are needed to keep the nucleus stable • No nuclei are stable when Z > 83 Radioactivity • Radioactivity is the spontaneous emission of radiation • Radioactivity is the result of the decay (disintegration) of unstable nuclei • Three types of radiation can be emitted • 1) Alpha particles: 4He nuclei) • 2) Beta particles: either electrons or positrons (a positron is the antiparticle of the electron, similar to the electron except its charge is +e) • 3) Gamma rays: high energy photons Penetrating Ability of Particles • Alpha particles: barely penetrate a piece of paper • Beta particles: can penetrate a few mm of aluminum • Gamma rays: can penetrate several cm of lead The Decay Constant • The number of particles that decay in a given time is proportional to the total number of particles in a radioactive sample ΔN = - λ N Δt • λ: the decay constant; determines the rate at which the material will decay • The decay rate or activity, R, of a sample is defined as the number of decays per second N R N t Decay Curve • The decay curve follows the equation N = No e- λt • Another useful parameter is the half-life defined as the time it takes for half of any given number of radioactive nuclei to decay T1 2 ln 2 0.693 Decay Curve N N 0e t 1 e T1 / 2 2 N0 N 0 e T1 / 2 2 e T1 / 2 2 T1/ 2 ln 2 T1 2 ln 2 0.693 Units • The unit of activity, R, is the Curie, Ci: 1 Ci = 3.7 x 1010 decays/second • The most commonly used units of activity are the mCi and the µCi • The SI unit of activity is the Becquerel, Bq: 1 Bq = 1 decay/second (therefore, 1 Ci = 3.7 x 1010 Bq) Maria Skłodowska-Curie 1867 – 1934 Antoine Henri Becquerel 1852 – 1908 Chapter 29 Problem 24 A building has become accidentally contaminated with radioactivity. The longest-lived material in the building is strontium-90. (The atomic mass of is 89.907 7.) If the building initially contained 5.0 kg of this substance, and the safe level is less than 10.0 counts/min, how long will the building be unsafe? Decay – General Rules • When one element changes into another element, the process is called spontaneous decay or transmutation • The sum of the mass numbers, A, must be the same on both sides of the equation • The sum of the atomic numbers, Z, must be the same on both sides of the equation • Conservation of mass-energy and conservation of momentum must hold Alpha Decay • When a nucleus emits an alpha particle it loses two protons and two neutrons (N decreases by 2; Z decreases by 2; A decreases by 4) • Symbolically A Z X ZA42Y 24He • X: parent nucleus; Y: daughter nucleus • Example: decay of 226 Ra (half life is 1600 years) 226 222 4 88 Ra 86 Rn 2 He • Excess mass is converted into kinetic energy • Momentum of the two particles is equal and opposite Beta Decay • During beta decay, the daughter nucleus has the same number of nucleons as the parent, but the atomic number is changed by one A A • Symbolically: Z X Z 1Y e A Z X Y e A Z 1 • The emission of the electron is from the nucleus, which contains protons and neutrons • The process occurs when a neutron is transformed into a proton and an electron • The energy must be conserved: the energy released in the decay process should almost all go to kinetic energy of the electron (KEmax) Beta Decay • Experiments showed that few electrons had this amount of kinetic energy • To account for this “missing” energy, Pauli proposed the existence of another particle, which Fermi later named the neutrino • Properties of the neutrino: • Zero electrical charge • Mass much smaller than the electron, probably not zero • Spin of ½ • Very weak interaction with matter Beta Decay • Symbolically A Z X Z A1Y e A Z X Z A1Y e • is the symbol for the neutrino; antineutrino is the symbol for the • Therefore, in beta decay, the following pairs of particles are emitted: either an electron and an antineutrino or a positron and a neutrino Gamma Decay • Gamma rays are given off when an excited nucleus “falls” to a lower energy state (similar to the process of electron “jumps” to lower energy states and giving off photons) • The gamma ray photons are very high in energy relative to light • The excited nuclear states result from “jumps” made by a proton or neutron • The excited nuclear states may be the result of violent collision or more likely of an alpha or beta emission Gamma Decay • Example of a decay sequence 12 5 B C * e 12 6 C*126C 12 6 • The first decay is a beta emission • The second step is a gamma emission • The C* indicates the Carbon nucleus is in an excited state • Gamma emission doesn’t change either A or Z Natural Radioactivity • There are unstable nuclei found in nature, which give rise to natural radioactivity • Nuclei produced in the laboratory through nuclear reactions exhibit artificial radioactivity • Three series of natural radioactivity exist (see table 29.2): 1) Uranium 2) Actinium 3) Thorium (processes through a series of alpha and beta decays and ends with a stable isotope of lead, 208Pb) Nuclear Reactions • Structure of nuclei can be changed by bombarding them with energetic particles • These changes are called nuclear reactions • As with nuclear decays, the atomic numbers and mass numbers must balance on both sides of the equation • E.g., alpha particle colliding with nitrogen: 4 2 He 14 7 N X 11H X 17 8 X 17 8 O • Balancing the equation allows for the identification of X, so the reaction is 4 2 He 14 7 N 17 8 O 11H Chapter 29 Problem 39 Identify the unknown particles X and X’ in the following 1 nuclear reactions: X + 42 He → 24 + Mg 12 0n 235 92 U + 01 n → 90 38 Sr + X + 2 01 n 2 11 H → 21 H + X + X’ Answers to Even Numbered Problems Chapter 28: Problem 4 ρn/ρa = 8.6 × 1013 Answers to Even Numbered Problems Chapter 28: Problem 8 a) 1.9 × 10-15 m b) 7.4 × 10-15 m Answers to Even Numbered Problems Chapter 28: Problem 10 (a) 1.11 MeV/nucleon (b) 7.07 MeV/nucleon (c) 8.79 MeV/nucleon (d) 7.57 MeV/nucleon Answers to Even Numbered Problems Chapter 28: Problem 18 (a)8.06 d (b) It is probably 13153I Answers to Even Numbered Problems Chapter 28: Problem 20 2.29 g Answers to Even Numbered Problems Chapter 28: Problem 38 4 4 He He, 2 2 Answers to Even Numbered Problems Chapter 28: Problem 42 a) 105B b) –2.79 MeV