* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Aminoglycoside - 123seminarsonly.com
Prescription costs wikipedia , lookup
Discovery and development of antiandrogens wikipedia , lookup
Discovery and development of tubulin inhibitors wikipedia , lookup
Drug interaction wikipedia , lookup
Neuropharmacology wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Chloramphenicol wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Discovery and development of cephalosporins wikipedia , lookup
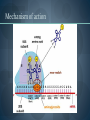
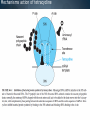
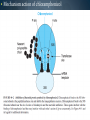
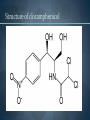
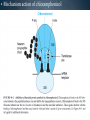
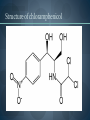
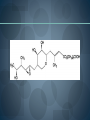
WELCOME OVERVIEW OF AMINOGLYCOSIDES AND OTHER PROTEIN SYNTHESIS INHIBITORS Presented By Maruf Kamal (2008-3-70-006) Sabbir Ahmed (2008-3-70-007) Md. Tanvir Rahman (2009-1-70-018) Introducton: An aminoglycoside is a molecule or a portion of a molecule composed of amino-modified sugars. Several aminoglycosides function as antibiotics that are effective against certain types of bacteria. They include amikacin, arbekacin, gentamicin, kanamycin, neomycin, netilmicin, paromomycin, rhodostreptomycin, streptomycin, tobramycin, and apramycin. History: Aminoglycoside Year Source organism streptomycin 1944 Streptomyces griseus neomycin 1949 Streptomyces fradiae kanamycin 1957 Streptomyces kanamyceticus paromomycin 1959 Streptomyces rimosus spectinomycin 1962 Streptomyces spectabilis gentamicin 1963 Micromonospora purpurea tobramycin 1968 Streptomyces tenebrarius sisomicin 1972 Micromonospora inyoensis amikacin 1972 semisynthetic derivative of kanamycin netilmicin 1975 semisynthetic derivative of sisomicin History: The first aminoglycoside, streptomycin, was isolated from Streptomyces griseus in 1943. Neomycin, isolated from Streptomyces fradiae, had better activity than streptomycin against aerobic gram-negative bacilli but, because of its formidable toxicity, could not safely be used systemically. Gentamicin, isolated from Micromonospora in 1963, was a breakthrough in the treatment of gram-negative bacillary infections, including those caused by Pseudomonas aeruginosa. Other aminoglycosides were subsequently developed, including amikacin (Amikin), netilmicin (Netromycin) and tobramycin (Nebcin), which are all currently available for systemic use Nomenclature Aminoglycosides that are derived from bacteria of the Streptomyces genus are named with the suffix -mycin, whereas those that are derived from Micromonospora are named with the suffix -micin. This nomenclature system is not specific for aminoglycosides. For example, vancomycin is a glycopeptide antibiotic and erythromycin, which is produced from the species Saccharopolyspora erythraea (previously misclassified as Streptomyces) along with its synthetic derivatives clarithromycin and azithromycin, is a macrolide. All differ in their mechanisms of action, however. Physical and chemical properties: They are water-soluble due to their polar groups (hydroxyl and amine groups), stable in solution and more active at alkaline than at acid PH. Aminoglycosides frequently exhibit synergism with β-lactams or vancomycin. However, aminoglycosides may complex with β- lactam drugs, resulting in loss of activity and they should not be mixed together for administration. Specific Agents Amikacin (Amikin®), Gentamicin (Garamycin®), Kanamycin (Kantrex®), Neomycin, Netilmicin (Netromycin®), Streptomycin, Tobramycin (Nebcin®) Structure of Aminoglycosides Mechanism of action bactericidal; aminoglycosides bind to the 30S subunit of the bacterial ribosome, interfering with the binding of fMet-tRNA and therefore the formation of the initiation complex. Binding to the 30S subunit may also cause misreading of mRNA codons β-lactams, vancomycin facilitate uptake by Grampositive organisms resistance: via plasmid-mediated aminoglycosidemodifying enzymes Mechanism of action Pharmacokinetics poor oral absorption volume of distribution approximates the extracellular space (about 0.26 L/kg) (larger in cystic fibrosis patients, about 0.35 L/kg) tissue distribution variable (poor CNS penetration) negligible metabolism renally eliminated (filtered, with a small amount of proximal reabsorption) elimination half-life: 2-3 hours (if renal function normal) Pharmacodynamics concentration-dependent killing postantibiotic effect (concentration-dependent) SARs of Aminoglycosides Crucial for broad spectrum activity Primary target for inactivating enzymes Congeners with amino groups at 2' and 6' are especially active SARs of Aminoglycosides Methylation of these amines does not alter activity,decreases inactivation Hydroxyls at the 3' or 4‘ position are not critically important SARs of Aminoglycosides Modifications compromise antibacterial activity One exception is amikacin with its aminohydroxybutyrate SARs of Aminoglycosides Substitution pattern is somewhat more flexible Only real requirement is the amine at the 3" position. Spectrum of activity Aminoglycosides are classified as broad-spectrum antibiotics, they used for treatment of serious systemic infections caused by Aerobic Gm –ve bacilli. Aerobic Gm –ve and Gm +ve cocci (with the exception of Staphylococci) tend to be less sensitive to aminoglycosides and thus the β-lactam and other antibiotics tend to be preferred for the treatment of infections caused by these organisms. Spectrum of activity broad gram-negative spectrum including P. aeruginosa gram-positive: synergistic in combination with ßlactams, glycopeptides anaerobes: negligible activity amikacin: Nocardia, MAI, certain rapid-growing mycobacteria, gentamicin-resistant gram-negative bacilli streptomycin: multidrug-resistant tuberculosis, tularemia, plague Adverse reactions nephrotoxicity proximal acute tubular necrosis (ATN) → ↓ GFR likely related to inhibition of intracellular phospholipases in the proximal tubule tends to be reversible associated factors: hypotension, dehydration, duration of therapy, concomitant liver disease, advanced age, other nephrotoxins (vancomycin) nephrotoxicity correlates with drug accumulation in the renal cortex Aminoglycoside accumulation in critically ill surgical patients Toxicity 1- Nephrotoxicity 2- Ototoxicity 3- Neurotoxicity 4- Neuromuscular blockade Additional adverse reactions with administration of aminoglycosides may include: nausea, vomiting, anorexia, rash, and urticaria. Toxicity ototoxicity vestibulotoxic and cochleotoxic generally irreversible difficult to assess high tone frequencies affected first neuromuscular blockade rare but potentially serious enhanced by conditions or drugs affecting the NM junction (e.g., myasthenia gravis, succinylcholine) can be treated with calcium Contraindications: Aminoglycosides should not be given to patients requiring long term therapy because of the potential for ototoxicity and nephrotoxicity. These drugs are contraindicated in patients with: - Preexisting hearing loss - Myasthenia gravis - Parkinsonism - During lactation or pregnancy. The aminoglycosides are used cautiously in patients with renal failure, in the elderly and in patients with neuromuscular disorders. Drug interactions: Administration of aminoglycosides with the cephalosporins may increase the risk of nephrotoxicity. When the aminoglycosides are administered with loop diuretics there is an increase the risk of ototoxicity (irreversible hearing loss). There is an increased risk of neuromuscular blockage (paralysis of the respiratory muscles) if the aminoglycosides are given shortly after general anesthetic (neuromuscular junction blockers). Drug interactions: Increased risk of nephrotoxicity and ototoxicity when aminoglycosides given with vancomycin. Increased risk of nephrotoxicity when aminoglycosides given with colistin. Aminoglycosides antagonize effects of neostigmine. Streptomycin sulfate NH H2N NH HO OH Streptidine OH NH H2N O O CHO NH L-Streptose CH3 OH O HO HO O NHCH3 N-Methyl-L- Glucosamine OH Streptomycin Neomycin sulfate Neosamine C CH2NH2 6 5 O 1 NH2 2 3 4 6 1 NH2 HO NH2 O 5 OH 5 Deoxystreptamine CH2OH O 6 O CH2NH2 4 1 5 HO O 3 2 1 3 HO NH2 O OH Neosamine C D-Ribose HO 3 Neomycin C Kanamycin sulfate 6` Ring I CH2R1 5` HO O Ring II 1` NH2 2 3 4 6 1 O 5 O HO 3` HO R2 HOCH 2 5`` HO 4`` H2 N NH2 1`` OH O 2`` Ring III Kanamycin A R1=NH2, R2=OH Kanamycin B R1=NH2, R2=NH2 Kanamycin C R1=OH, R2=NH2 Amikacin Ring I CH2NH2 6` 5` O HO 3` HO Ring II 1` NH2 2 3 4 6 1 OH O 5 O HO HOCH 2 5`` HO 4`` H2N O 1`` OH 2`` Ring III Amikacin O OH NH-C-C-CH 2CH2NH2 H Gentamicin sulfate Netilmicin sulfate 6` CH 2OH 5` 4` Ring I 2` 3` O NH2 1` H2 N 3 4 O 6 2 Ring II 1 NHR 5 O HO 5`` CH3 4`` 3`` O 1`` OH Ring III 2`` NHCH 3 OH Sisomicin R= H Netilmicin R= C2H5 Other Protein Synthesis Inhibitors Tetracycline Chloramphenicol Macrolides Mupirocin Quinolones Mechanisms action of tetracycline Commercially available tetracyclines First generation (Dose intervals shorter) Chlorotetracycline Oxytetracycline Tetracycline Dmeclocycline • Second Generation (Dose interval longer) Minocycline Methacycline Doxycycline • Third Generation Glycylcycline Mechanism action of chloramphenicol Structure of cloramphenicol MUPIROCIN Mupirocin is active against many gram-positive and selected gram-negative bacteria. It has good activity against S. pyogenes and methicillin-susceptible and methicillin-resistant strains of S. aureus. It is bactericidal at concentrations achieved with topical application. Mupirocin inhibits bacterial protein synthesis by reversible inhibition of Ile tRNA synthase. There is no cross-resistance with other antibiotic classes. Clinically insignificant, low-level resistance results from mutations of the gene encoding Ile tRNA synthase or an extra chromosomal copy of a gene encoding a modified Ile tRNA synthase. High-level resistance is mediated by a plasmid or chromosomal copy of a gene encoding a “bypass” synthetase that binds Mupirocin poorly. Mechanism action of chloramphenicol Structure of chloramphenicol Mode of action of Macrolides Examples of Macrolides Erythromycin Clarithromycin Roxithromycin Azithromycin Mupirocin is available as a 2% cream or ointment for dermatologic use and as a 2% ointment for intranasal use. The dermatological preparations are indicated for treatment of traumatic skin lesions and impetigo secondarily infected with S. aureus or S. pyogenes. Systemic absorption through intact skin or skin lesions is minimal. Any Mupirocin absorbed is rapidly metabolized to inactive monic acid. Mupirocin is effective in eradicating S. aureus carriage. The consensus is that patients who may benefit from Mupirocin prophylaxis are those with proven S. aureus nasal colonization plus risk factors for distant infection or a history of skin or soft tissue infections. Mupirocin may cause irritation and sensitization and contact with the eyes should be avoided. Systemic reactions to Mupirocin occur rarely, if at all. Application of the ointment to large surface areas should be avoided in patients with renal failure to avoid accumulation of polyethylene glycol from the ointment. Examples of Quinolones Nalidixic acid Ciprofloxacin Levofloxacin Glatifloxacin Norfloxacin Sparfloxacin Fluroquinolone THANK YOU