* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Aminoglycosides
Discovery and development of angiotensin receptor blockers wikipedia , lookup
Discovery and development of antiandrogens wikipedia , lookup
Magnesium transporter wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
Neuropharmacology wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
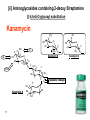
Discovery and development of cephalosporins wikipedia , lookup
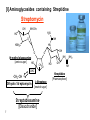
Lecture 6 Medicinal Chemistry 1 (PC 509) Prof. Dr/ Ghaneya Sayed Hassan [email protected] 1 Aminoglycosides Warning Patients treated with aminoglycosides should be under close clinical observation because of the potential toxicity associated with their use. They are antibiotics obtained from m.o. in the soil: (1) Streptomyces [end with suffix mycin]. (2) Micromonospora [end with suffix micin]. Prototype of this class [first one used] is Streptomycin. M.O.A: [inhibition of bacterial protein biosynthesis] Irreversibly binding the 30S ribosomal subunit of m.o. through specific receptor protein inhibit initiation of protein synthesis cause misreading of genetic code on recognition region of ribosome insertion of wrong amino acids non-functional protein [antisense protein] destruction of cell membrane bactericidal action. 2 General properties: 1) They are BACTERICIDAL, effective only ≠ aerobic bacteria [they are inactive ≠ anaerobic bacteria that transport through cell membrane is energy-requiring process that is oxygen-dependent]. 2) They are limited use and for only severe G-ve infections due to their toxicity [cause ototoxicity & nephrotoxicity by action on 8th cranial nerve]. 3) Freely water soluble used by injection. But Neomycin must not be given parentrally [ toxicity], only used topically or orally in hepatic coma to intestinal bacterial population. 4) Very poorly absorbed from GIT [highly polar compounds] when used orally it's intended for local GIT infections. But given I.M. for systemic infections. There is no oral form available as aminoglycosides are not absorbed orally 3 5) Aminoglycosides + -lactams synergism but must not be combined in the same solution [should be given into different tissue comportments, as given each preparation one in each arm] they are chemically incompatible. Inactivate if put with β-lactam antibiotics in same preparation Nacylation by β-lactam ring inactivation of both antibiotics. COOH NH H2N RCOHN Sugar O NH2 HO O + O RCOHN O N COOH Sugar HN Sugar O HO NH2 O GenamicnC-2a Active B-Lactam Antibiotic Active Inactive Sugar 6) Aminoglycosides can exacerbate weakness in patients with myasthenia gravis ,and use is therefore avoided in these patients 4 Resistance: There are three mechanisms: (1) Reduced uptake or decreased cell permeability: Permeability defect: by outer membrane change active transport into cell drug can't reach ribosome. (2) Alterations at the ribosomal binding sites: Lack of specific protein receptor on 30 S subunit lower affinity of binding site to aminoglycosides. (3) Production of aminoglycoside modifying enzymes: Several of the aminoglycoside antibiotics can be inactivated by enzymes which occur in gram-negative bacteria carrying R-factors. R-factor genes mediated the formation of aminoglycoside-inactivating enzymes 1-Aminoglycoside 6'-N-acetyltransferase: 4' Ad 6' HO HO enzymes make acetylation of NH2 group. (ANT) 3' Phos 2-O-Phosphoryl transferase: enzymes make phosphorylation of OH group. (APT) 3- O-Adenyl transferase : enzymes make O-adenylation. (AAT) Inositol (Cyclhexanhexaol) 5 NH2 5' Ac O 2' 1' H OH O 4 Chemical properties: • Pharmacophoric unit is 1,3-diamino Inositol moiety “aminocyclitol unit”. • From this moiety ; we have 3 pharmacophoric groups : Streptidine 2-deoxy streptamine H2N H2N NH HN 3 HO H3C-HN HO 2 OH HO 1 OH NH2 OH HO NH2 HO NH Spectinamine OH NH CH3 HO OH NH (1) Alcoholic functions of these moieties [OH] make glycosidic bonds with characteristic amino sugars [aminoglycosides]. (2) They are usually formed of 3 rings, freely soluble in water [why?] 6 They are basic [due to amino group], form acid addition salts. (3) [I] Aminoglycosides containing Streptidine Streptomycin NH-CH3 OH H2N HO NH O HN HOH2C O N-methyl glucosamine [amino sugar] HO OHC -CH2-OH Dihydro Streptomycin CH3 O NH HO OH NH2 NH Streptidine L-Streptose [neutral sugar] Streptobiosamine [Disaccharide] 7 OH O [Pharmacophore] • Produced by Streptomyces griseus. • Basic, water soluble [available as sesquisulfate salt which is quite soluble in water]. • With hydrophilic nature poorly absorbed from GIT [usually taken I.M.] M.O.A: Inhibition of protein synthesis + misreading of mRNA & membrane damage. Diffuse across outer membrane of T.B. penetrate cytoplasmic membrane through electron-dependent process. Uses: (1) Bactericidal [I.M limit its use in long-course therapy] (2) Used in combination for T.B. + other infections. 8 SAR: (1) Reduction of aldehyde to alcohol Dihydrostreptomycin [similar activity but with delayed severe deafness]. (2) Oxidation of aldehyde to carboxyl or conversion to Schiff's base [oxmie, semicarbazone, phenyl hydrazone] inactive (3) Oxidation of Methyl group in -streptose to methylene hydroxyl active but with no advantage over STM. (4) Demethylation or replacement of amino methyl in glucosamine part with larger alkyl group activity. (5) Removal or modification of either guanidine 9 activity. ► Produced by fermentation of Sterptomyces griseus & related soil m.o. ► Introduced primarily for treatment of T.B.[ may be due to presence of two guanido moieties]. But replaced now by other antibiotics as Rifampin. Dihydro streptomycin: Formed by replacing aldehydic group in L-streptose moiety by alchoholic group [CH2OH] With greater probability > streptomyces to cause delayed deafness. 10 [ii] Aminoglycosides containing 2-deoxy Streptamine [i] 4,6-di-O-glycosyl substitution Kanamycin NH2 4' 6' Ad HO HO 3' HO HO NH2 5' Ac H O 1' H OH O 4 HN 2 HO HO 6'' 2 3 6 5 1 NH2 O 5'' HO 4'' H2N 11 HO Kanmycin B Phos Kanmycin A O NH2 O 2' OH 1'' O 3'' Glycosidic linkage OH 2'' O H HO NH2 Kanmycin C O ■ Isolated from Streptomyces kanamyceticus mixture of Kanamycin A, B & C. [according to D-glucose attached to 4-position of 2-deoxy streptamine]. ■ Commercially available Kanamycin is pure Kanamycin A [the least toxic of them]. ■ Chemically stable within pH of 2-11, very stable to heat [sterilized by autoclaving]. ■ Unstable to R-factor enzymes: [giving INACTIVE compounds] 3'-OH Phosphorylation. 4'-OH Adenylation. 6'-NH2 Acetylation. ■ To improve its activity SEMI-SYNTHETIC PRODUCTS by: (1) Adding functional group that prevents enzymatic attack. (2) Removal of susceptible functional groups which are not important for activity. 12 Semi-synthetic aminoglycosides Amikacin [Amikin®] 4' X Ad Phos X HO HO 6' 3' NH2 5' O 2' 1' H OH O 4 H N 2 HO 6 5 1 NH O 1'' HO O HO H2N 13 2 3 OH 2'' O OH C CH CH2 CH2 NH2 L-AHBA The side chain protects amikacin from attack by steric hindrance or folding L--Amino-α-Hydroxy Butyric Acid Semi-synthetic analogue of Kanamycin A produced by acylation of 1-amino group with L-AHBA [L--Amino-α-Hydroxy Butyric Acid]. Advantages: (1) Less toxic than Kanamycin or Gentamicin. (2) L-AHBA binding to R-factor mediated enzymes prevent adenylation at C4' are phosphorylation at C3‘ potency & broaden spectrum. Amikacin is resistant to many R-factor mediated enzymes due to the presence of L-AHBA [L--Amino-α-Hydroxy Butyric Acid]. Uses: serious infections by bacteria those are resistant to other aminoglycosides 14 Gentamicin [Garamycin®] H3C X Ad Phos 4' X NH CH3 CH 6' 4' Ac 5' 3' NH2 H2C 6' 1' H NH2 O 4 H N 2 HO 3' 2 3 6 5 4' 5' O 2' H3C CH 6' 5' O 2' NH2 1 NH 2 NH2 1' H O Gentamicin C1a 3' O 2' NH2 1' H O Gentamicin C2 O Gentamicin C1 1'' O 3HC HO HN OH 2'' CH3 Garosamine Suger: specific for all Gentamicins Produced by fermentation of Micromonspora purpurea & other related soil m.o. [mixture of compounds] 15 Uses: 1- Has significant activity ≠ Pseudomonas aeroginosa infections [in burns, pneumonias & UTI], also, prevent fouling of soft contact lenses. 2- It is not effective for gonorrhea or chlamydia infections. 3- Gentamicin is also used in molecular biology research as an antibacterial agent in tissue and cell culture, to prevent contamination of sterile cultures. Gentamicin is one of the few heat-stable antibiotics that remain active even after autoclaving ,which makes it particularly useful in the preparation of some microbiological growth media 16 [iii] Aminoglycosides with Spectinamine Spectinomycin Spectinamine Ring Spectinamine Ring OH CH3 OH O O HN OH O O CH3 H3C O NHCH3 OH O OH O NH CH3 O OH NH CH3 Produced by fermentation of Streptomyces spectabilis. Composed of Spectinomine moiety + bicyclic moiety fused at 4-OH & 5-OH. [with only one sugar]. The interaction of Spectinomycin with the 30S-ribosomal subunit is reversible. Spectinomycin is an aminocyclitol antibiotic that is structurally related to aminoglycosides. It lacks amino sugars. 17 M.O.A: Interfere with binding t-RNA to ribosomes interfere with initiation of protein synthesis [as Streptomycin], BUT, NOT cause misreading of messenger [≠ Streptomycin] Bacteriostatic NOT Bactericidal effect. Uses: (1) Alternative to penicllins, that it not cause serious otoor nephrotoxicity. (2) It's the drug of choice in treatment of GONORRHEA cause by penicillinase-producing N. gonorrhea. 18