* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Blood Drugs
Psychedelic therapy wikipedia , lookup
Discovery and development of proton pump inhibitors wikipedia , lookup
Discovery and development of cyclooxygenase 2 inhibitors wikipedia , lookup
Discovery and development of antiandrogens wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
NK1 receptor antagonist wikipedia , lookup
Prescription costs wikipedia , lookup
Discovery and development of neuraminidase inhibitors wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Metalloprotease inhibitor wikipedia , lookup
Pharmacognosy wikipedia , lookup
Discovery and development of angiotensin receptor blockers wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
Drug interaction wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Psychopharmacology wikipedia , lookup
Neuropharmacology wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Discovery and development of direct Xa inhibitors wikipedia , lookup
Discovery and development of direct thrombin inhibitors wikipedia , lookup
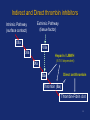
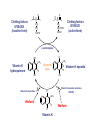
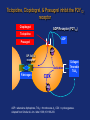
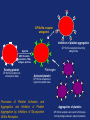
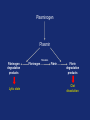
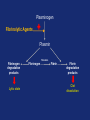
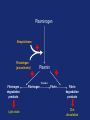
Drugs used in the coagulation disorder Haemostasis Haemostasis is a physiological process that aims to maintain the blood in a fluid state under normal condition It refers to the normal response of the vessel to injury Haemostatic mechanisms involve a complex interplay b/w three components: 1) Blood cells 2) Vascular wall 3) Coagulation factors Disturbances of haemostasis result in several pathological conditions Haemostasis A wound causes vasoconstriction, accompanied by: 1) Adhesion and activation of platelets 2) Fibrin formation Platelets first adhere to macromolecules in the subendothelial regions of the injured blood vessel, where they become activated Adherent platelets release substances that activate nearby platelets, thereby recruiting them to the site of injury Haemostasis Activated platelets then aggregate to form the primary hemostatic plug In addition to triggering platelet adhesion and activation, vessel wall injury also exposes tissue factor (TF), which initiates the coagulation system Physical trauma to the vascular system, such as a puncture or a cut, initiates a complex series of interactions between platelets, endothelial cells, and the coagulation cascade Drugs affecting haemostasis Drug therapy to promote haemostasis is rarely Drug therapy to treat or prevent thrombosis or thromboembolism, is extensively used because such diseases are common as well as serious Drugs affect haemostasis and thrombosis in three distinct ways, by affecting: 1) Blood coagulation (fibrin formation) 2) Platelet function 3) Fibrin removal (fibrinolysis) I. Anticoagulants Blood coagulation A sequential process of chemical reactions involving clotting factors, phospholipids, and calcium ions. This leads the blood to form solid clots Blood coagulation is considered as a series of steps in which plasma inactive enzyme precursors (proenzymes) are transformed into active enzymes Two main pathways have been described for blood coagulation: the intrinsic and the extrinsic pathways Blood coagulation Platelet activation and coagulation do not normally occur within an intact blood vessel Thrombosis is prevented by several regulatory mechanisms that require a healthy vascular endothelium Under most circumstances, TF exposed at sites of vessel wall injury initiates coagulation via the extrinsic pathway Blood coagulation This results in a burst of thrombin (factor IIa) generation Thrombin then converts fibrinogen to fibrin, which reinforces the platelet aggregate and anchors it to the vessel wall. In addition, because it serves as a potent platelet agonist, thrombin also amplifies platelet activation and aggregation Later, as wound healing occurs, the platelet aggregates and fibrin clots are degraded Extrinsic Pathway Intrinsic Pathway TF VII XII XI IX XIIa X TF-VIIa XIa IXa Prothrombin Xa AT Va Thrombin AT Fibrinogen Fibrin Blood coagulation Control of the coagulation system is regulated by a series of protease inhibitors: protein C and S, antithrombin III (ATIII), and tissue factor pathway inhibitor (TFPI) Antithrombin is a glycosylated, single-chain polypeptide composed that is synthesized in the liver Antithrombin inhibits clotting factor proteases, especially thrombin (IIa), IXa, and Xa, by forming equimolar stable complexes with them Anticoagulants They inhibit the formation of fibrin by actions on the coagulation phase They do not lyse clots or affect the fibrinolytic pathway They can: I. Indirect thrombin inhibitors II. Direct thrombin inhibitors III. Oral direct factor Xa inhibitors IV. Warfarin and other coumarin anticoagulants Indirect and Direct thrombin inhibitors Intrinsic Pathway (surface contact) Extrinsic Pathway (tissue factor) XIIa VIIa XIa Heparin / LMWH (AT-III dependent) IXa Xa Direct antithrombin Thrombin (IIa) Thrombin-Fibrin clot 15 Indirect Thrombin Inhibitors Agents: unfractionated heparin (UFH), molecular-weight heparin (LMWH), fondaparinux lowand The indirect thrombin inhibitors are so-named because their antithrombotic effect is exerted by their interaction with a separate protein, antithrombin III (ATIII) and enhance its inactivation of factor Xa Heparin & LMWHs Two types of heparin are used clinically: 1.Older standard (unfractionated/ molecular weight) heparin high 2.Newer type, called low-molecular-weight heparin (LMWH), is derived from unfractionated heparin The two classes are similar but not identical in their actions and PKs Heparin & LMWHs I. Unfractionated heaprin A mixture of highly electronegative acidic mucopolysaccharides found in the secretory granules of mast cells Commonly extracted from porcine intestinal mucosa or bovine lung Heparin & LMWHs II. Low molecular weight heprin (LMWH) Incude: enoxaparin, dalteparin, and tinzaparin Low-molecular-weight fragments produced by chemical depolymerization and extraction of standard heparin Advantages: greater bioavailability than standard heparin, a longer-lasting effect, & more predictable relationship between anticoagulant response and dose Heparin & LMWHs- MOA Heparin, LMWHs, and fondaparinux have no intrinsic anticoagulant activity The active heparin molecules bind tightly to antithrombin and cause a conformational change in this inhibitor The conformational change of antithrombin exposes its active site for more rapid interaction with the proteases (the activated clotting factors) This conformational change accelerates the rate of factor Xa inhibition by 1000-fold Heparin & LMWHs- MOA Unfractionated heparin binds to antithrombin III and induces a conformational change that accelerates the interaction of antithrombin III with the coagulation factors- most importantly, thrombin (Factor IIa) and Factor Xa To potentiate thrombin inhibition, heparin must simultaneously bind to antithrombin and thrombin Only heparin chains composed of at least 18 saccharide units (molecular weight 5,400 Da) are of sufficient length to perform this bridging function Heparin & LMWHs- MOA LMWHs complex with antithrombin III and inactivate Factor Xa but have less effect on thrombin. They are too short to bridge antithrombin and thrombin together Unfractionated Heparin Thrombin (IIa) (FXa) AT AT LMWH (FXa) AT Heparin and LMWHs-PKs Heparin and LMWHs are not absorbed through the GI mucosa and therefore must be given parenterally Heparin is given by continuous intravenous infusion, intermittent infusion every 4-6 hours, or subcutaneous injection every 8-12 hours Heparin has an immediate onset of action when given intravenously In contrast, there is considerable variation in the bioavailability of heparin given subcutaneously, and the onset of action is delayed 1-2 hours Heparin and LMWHs- PKs LMWH are absorbed more uniformly after subcutaneous injection The t1/2 of heparin in plasma depends on the dose administered (t1/2 1-5 hours) LMWHs have longer biological half-lives than heparin (t1/2 4-6 hours) Heparin and LMWHs-PKs Heparin appears to be cleared and degraded primarily by the reticuloendothelial system; a small amount of undegraded heparin also appears in the urine LMWHs are cleared almost exclusively by the kidneys, the drugs can accumulate in patients with renal impairment, which can lead to bleeding Comparison of the Features of Heparin, LMWH, and Fondaparinux Feature Source Molecular weight (Da) Target Bioavailability (%) t1/2 (h) Renal excretion Antidote effect Thrombocytopenia Heparin Biological 15,000 Xa and IIa 30 1 No Complete <5% LMWH Biological 5000 Xa 90 4 Yes Partial <1% Fondaparinux Synthetic 1500 Xa 100 17 Yes None <1% Heparin and LMWHs Heparin is prescribed on a unit (IU) rather than milligram basis The dose must be determined on an individual basis Therapy routinely is monitored by the activated partial thromboplastin time (aPTT or PTT) & anti-Xa activity Heparin and LMWHs Heparin and LMWH are used during coronary balloon angioplasty with or without stent placement to prevent thrombosis In contrast to warfarin, heparin and LMWH do not cross the placenta and have not been associated with fetal malformations; therefore, these are the drugs of choice for anticoagulation during pregnancy Adverse effects 1. Bleeding complications: The chief complication of heparin therapy is hemorrhage Protamine sulfate is used routinely to reverse the anticoagulant effect of heparin: protamine is a highly basic peptide that combines with heparin as an ion pair to form a stable complex devoid of anticoagulant activity Adverse effects 2. Hypersensitivity reactions: Include chills, fever, urticaria, or anaphylactic shock Cause: heparin preparations are obtained from porcine sources and, therefore, may be antigenic 3. Osteoporosis: has been reported with long-term (6 months or more) treatment 4. Hypoaldosteronism is uncommon, but increases with prolonged treatment Adverse effects 5. Heparin-induced thrombocytopenia (HIT): It is a common abnormality among hospital patients receiving heparin HIT (platelet count <150,000/mL or a 50% decrease from the pretreatment value) occurs in 0.5% of medical patients 5-10 days after initiation of therapy with heparin The risk of HIT may be higher in individuals treated with UFH of bovine origin compared with porcine heparin and is lower in those treated exclusively with LMWH Adverse effects 5. Heparin-induced thrombocytopenia (HIT): Patients who develop HIT are treated by discontinuance of heparin and administration of a direct thrombin inhibitor Fondaparinux Pentasaccharide anticoagulants that is purely synthetically, derived with no variable biologic activity (i.e. requires less monitoring than heparin) It selectively inhibits only Factor selectively binding to antithrombin III Xa, by Clinical uses in the prophylaxis of DVT that could lead to PE in patients undergoing hip fracture surgery, hip replacement surgery, and knee replacement surgery Fondaparinux Fondaparinux is administered by subcutaneous injection, reaches peak plasma levels in 2 hours, and is excreted in the urine (t1/2 17 h) It should not be used in patients with renal failure Fondaparinux can be given once a day at a fixed dose without coagulation monitoring Fondaparinux appears to be much less likely than heparin or LMWH to trigger the syndrome of heparin-induced thrombocytopenia Oral direct factor Xa inhibitors Agents: rivaroxaban, apixaban, edoxaban, betrixaban They inhibits factor Xa, in the final common pathway of clotting Rivaroxaban is approved for the prevention of VTE following hip or knee surgery & for treating DVT or PE, and to reduce the risk of recurrent DVT and PE following initial treatment These drugs are administered as fixed doses and do not require monitoring Oral direct factor Xa inhibitors They have a rapid onset of action and shorter half-lives than warfarin They are excreted in part by the kidneys ADRs: predictable bleeding and nausea Direct thrombin inhibotrs (DTIs) 1) Parenteral direct thrombin inhibitor: hirudin, bivalirudin, Lepirudin, argatroban 2) Oral direct thrombin inhibitors: dabigatren etexilate mesylate Exert their anticoagulant effect by directly binding to the active site of thrombin, thereby inhibiting thrombin's downstream effects Parenteral direct thrombin inhibitors Clinical uses: I. Alternative to heparin primarily in patients with heparin induced thrompocytopenia II. Argatroban and pivalirudin: during percutaneous coronary interventions (PCI) in patients who have or are at risk for developing HIT Are administered intravenously and the dose being adjusted depending on the aPTT They can cause bleeding or hypersensitivity reactions (rash or fever) Oral direct thrombin inhibitors Dabigatran etexilate mesylate, is an orally active direct thrombin inhibitor It is licensed for prevention of VTE following hip or knee replacement and to reduce risk of stroke and systemic embolism with nonvalvular atrial fibrillation Advantages include: predictable PKs and bioavailability, which allow for fixed dosing and predictable anticoagulant response, and make routine coagulation monitoring unnecessary Oral direct thrombin inhibitors Dabigatran does interacting drugs not interact with P450- It has a very rapid onset and offset of action allow for immediate anticoagulation, thus avoiding the need for overlap with additional anticoagulant drugs ADRs: bleeding Vitamin K antagonists: warfarin Are derivatives of 4-hydroxycoumarin or indan1,3-dione, and they resemble vitamin K Coumarin anticoagulants block the gammacarboxylation of several glutamate residues in prothrombin and factors VII, IX, and X as well as the endogenous anticoagulant proteins C and S The blockade results in incomplete coagulation factor molecules that are biologically inactive Vitamin K antagonists- MOA Unlike heparin, the anticoagulant effects of warfarin are not observed until 8 to 12 hours after drug administration, but peak effects may be delayed for 72 to 96 hrs Figure 1.4 Warfarin and the vitamin K cycle. Clotting factors II/VII/IX/X (inactive form) H H N C O O H H N C CH2 CHCOOH COOH CH 2 CH 2 COOH Clotting factors II/VII/IX/X (active form) γ-carboxylase O OH CH3 Vitamin K hydroquinone R CH 3 Vitamin K Cycle Vitamin K epoxide R OH OH Vitamin K reductase O Vitamin K epoxide reductase (VKOR) O CH2 Warfarin R O Vitamin K Warfarin Warfarin The bioavailability of warfarin is nearly complete when the drug is administered orally, intravenously, or rectally Over 99% of warfarin is bound to plasma albumin, which may contribute to its small volume of distribution, its long half-life in plasma (36 hours), and the lack of urinary excretion of unchanged drug S- warfarin is metabolized principally by CYP2C9 Warfarin Warfarin is the most important oral anticoagulant and is of the most commonly prescribed drugs Clinical uses: I. Prevent the progression or recurrence of acute DVT or PE after initial heparin treatment II. Prevention of VTE gynecologic surgery during orthopaedic or III. Prophylactically, it is used in patients with acute MI, prosthetic heart valves, or chronic atrial fibrillation Warfarin The international normalized ratio (INR), as a means of standardizing the PT results, is the most recommended method for monitoring patients’ warfarin therapy The goal of warfarin therapy is an INR of 2 to 3 for most indications and 2.5 to 3.5 in patients with mechanical heart valves Warfarin- ADRs 1. Bleeding disorders: - The principal untoward reaction caused by warfarin treatment is hemorrhage - Therefore, it is important to frequently monitor and adjust the anticoagulant effect - Minor bleeding may be treated by withdrawal of the drug and administration of oral vitamin K1 - Severe bleeding requires that greater doses of the vitamin be given intravenously - Whole blood, frozen plasma, or plasma concentrates of the blood factors may also be employed to arrest hemorrhaging Warfarin- Drug & disease interactions Warfarin has a narrow therapeutic window, and interacts with other drugs, diet, and with disease states These interactions can be broadly divided into: I. Pharmacokinetic effects: mainly enzyme induction, enzyme inhibition, plasma protein binding II. and reduced Pharmacodynamic effects: vitamin K deficiency, hepatic disease that impairs synthesis of the clotting factors, and hypermetabolic states that increase catabolism of the vitamin K-dependent clotting factors PK & PD Drug and Body Interactions with Oral Anticoagulants Increased Prothrombin Time PK PD Amiodarone Drugs: Cimetidine Aspirin (high doses) Disulfiram Cephalosporins, Metronidazole1 third-generation Fluconazole1 Heparin Phenylbutazone1 Body factors: Sulfinpyrazone1 Hepatic disease TrimethoprimHyperthyroidism sulfamethoxazole 1Stereoselectively Decreased Prothrombin Time PK PD Barbiturates Drugs: Cholestyramine Diuretics Rifampin Vitamin K Body factors: Hereditary resistance Hypothyroidism inhibits the oxidative metabolism of the S-warfarin enantiomorph of racemic warfarin Warfarin Warfarin should never be used during pregnancy, because its teratogenic and can cause abortion and birth defects If anticoagulant therapy is needed during pregnancy, heparin or LMWH may be administered II. Antiplatelet Drugs Introduction The vascular endothelial cell layer lining blood vessels has an anticoagulant phenotype, and circulating blood platelets and clotting factors do not normally adhere to it to an appreciable extent Following disruption of the endothelial lining and exposure of blood to the subendothelial vessel wall, platelets adhere to and virtually cover the exposed collagen of the subendothelium This triggers a complex series of chemical reactions, resulting in platelet activation Introduction Activation of platelets results: 1) Activation of thromboxane A2 synthesis, from arachidonic acid within platelets, that causes platelets to change shape, release their granules, and aggregate 2) The release of platelet granules containing mediators, such as ADP and serotonin (5-HT), which stimulate aggregation 3) Conformational change in the IIb/IIIa receptor, enabling it to bind fibrinogen, which cross-links adjacent platelets, resulting in aggregation and formation of a platelet plug Platelet Aggregation Inhibitors Platelets provide the initial hemostatic plug at sites of vascular injury They also participate in pathological thromboses that lead to MI, stroke, and peripheral vascular thromboses These drugs decrease the formation or the action of chemical signals that promote platelet aggregation These drugs act by discrete mechanisms, and thus in combination their effects are additive or even synergistic Platelet Aggregation Inhibitors Antiplatelets include: 1) Aspirin and other NSAIDs 2) Glycoprotein IIb/IIIa receptor inhibitor 3) Antagonist of ADP receptors 4) Phosphodiesterase 3 inhibitors Sites of Action of Antiplatelet Therapy on Mechanisms of Platelet Activation and Aggregation Copyright restrictions may apply. Schulman, S. P. JAMA 2004;292:1875-1882. Sites of Action of Antiplatelet Therapy Clopidogrel ADP Receptor (P2Y12) Ticlopidine ADP Prasugrel ADP GP IIb/IIIa receptor Activation Collagen Thrombin TXA2 COX Fibrinogen TXA2 Abciximab Aspirin Eptfibatide Tirofiban ADP = adenosine diphosphate, TXA2 = thromboxane A2, COX = cyclooxygenase. Adapted from Schafer AI. Am J Med. 1996;101:199-209. Aspirin It inhibits the synthesis of thromboxane A2 by irreversible acetylation of the enzyme cyclooxygenase-1 (COX-1) Since platelets do not synthesize new proteins, the action of aspirin on platelet cyclooxygenase is permanent, lasting for the life of the platelet (7 to 10 days) Repeated doses of aspirin produce a cumulative effect on platelet function Aspirin Clinical uses: 1. Prophylactic treatment of transient cerebral ischemia 2. To reduce the incidence of recurrent MI 3. To decrease mortality in pre- and postmyocardial infarct patients Aspirin is frequently used in combination with other drugs having anticlotting properties (e.g. heparin and clopedogril) ADRs: increased incidence of hemorrhagic stroke as well as GIT bleeding, especially at higher doses Ticlopidine, Clopidogrel, & Prasugrel These drugs irreversibly block the ADP receptor on platelets and thereby prevent ADP-mediated platelet aggregation Platelets contain two GPCRs for ADP: 1) P2Y1: couples to the Gq–PLC–IP3–Ca2+ pathway and induces a shape change and aggregation 2) P2Y12 receptor couples to Gi and, when activated by ADP, inhibits adenylyl cyclase, resulting in lower levels of cyclic AMP and thereby less cyclic AMP–dependent inhibition of platelet activation Ticlopidine, Clopidogrel, & Prasugrel inhibit the P2Y12 receptor Clopidogrel ADP Receptor (P2Y12) Ticlopidine ADP Prasugrel ADP GP IIb/IIIa receptor Activation Fibrinogen Collagen Thrombin TXA2 COX TXA2 ADP = adenosine diphosphate, TXA2 = thromboxane A2, COX = cyclooxygenase. Adapted from Schafer AI. Am J Med. 1996;101:199-209. Ticlopidine, Clopidogrel, & Prasugrel Ticlopidine and clopidogrel arr effective in preventing transient ischemic attacks (TIAs) and ischemic strokes for patients with prior cerebral thrombotic event Ticlopidine, clopidogrel, & prasugrel are used in combination with aspirin following coronary stent implantation to decrease the incidence of stent thrombosis Ticlopidine, Clopidogrel, & Prasugrel Clopidogrel is approved for prophylaxis of thrombotic events in acute coronary syndrome and for prevention of atherosclerotic events following recent MI, stroke, or established peripheral arterial disease Ticlopidine, Clopidogrel, & Prasugrel Ticlopidine ADRs: 1.GIT: nausea, dyspepsia, and diarrhea 2.Hemorrhage 3.Leukopenia: detected by regular monitoring of the WBC count during the first 3 months of treatment 4.Thrombotic (TTP) thrombocytopenic purpura Ticlopidine, Clopidogrel, & Prasugrel Clopidogrel ADRs: - Clopidogrel has fewer ADRs than ticlopidine: less frequent thrombocytopenia and leukopenia - Clopidogrel is preferred over ticlopidine Prasugrel ADRs: the main side effect is bleeding which can be fatal Blockade of Platelet Glycoprotein IIb/IIIa Receptors Agents: abciximab, eptfibatide, & tirofiban These drugs target the platelet IIb/IIIa receptor complex By binding to GP IIb/IIIa, these drug reversibly block the binding of fibrinogen and von Willebrand factor; consequently, aggregation does not occur GP IIb/IIIa receptor antagonist Inhibition of platelet aggregation GP IIb/IIIa receptors bound by antagonists Agonist ADP, thrombin, epinephrine, TXA2, collagen, & others Resting platelet GP IIb/IIIa receptors in unreceptive state Fibrinogen Activated platelet GP IIb/IIIa receptors in ligand-receptive state Processes of Platelet Activation and Aggregation and Inhibition of Platelet Aggregation by Inhibitors of Glycoprotein IIb/IIIa Receptors Aggregation of platelets GP IIb/IIIa receptors are bound to fibrinogen, forming bridges between adjacent platelets Blockade of Platelet Glycoprotein IIb/IIIa Receptors These agents are administered parenterally (IV) Clinical uses: to prevent restenosis after coronary angioplasty and to decrease the incidence of thrombotic complications associated with acute coronary syndromes Major ADRs: bleeding III. Thrombolytic Drugs Introduction During plug formation, the fibrinolytic pathway is locally activated Plasminogen is enzymatically processed to plasmin (fibrinolysin) by plasminogen activators in the tissue Plasmin is a relatively nonspecific protease; it digests fibrin clots and other plasma proteins, including several coagulation factors Plasminogen Plasmin Thrombin Fibrinogen degradation products Lytic state Fibrinogen Fibrin Fibrin degradation products Clot dissolution Thromoblytic/Fibrinolytic Drugs Fibrinolytic agents include: 1) Tissue plasminogen activator: alteplase, erteplase, and tenecteplase 2) Streptokinase 3) Urokinases Either directly or indirectly lyse thrombi by catalyzing the formation of the serine protease plasmin from its precursor zymogen, plasminogen Plasminogen Fibrinolytic Agents Plasmin Thrombin Fibrinogen degradation products Lytic state Fibrinogen Fibrin Fibrin degradation products Clot dissolution Streptokinase Protein synthesized by streptococci that combines with the proactivator plasminogen It forms an active one-to-one complex with plasminogen This enzymatically active complex catalyzes the conversion of uncomplexed plasminogen to the active enzyme plasmin (fibrin plug hydrolysis) Plasminogen Streptokinase Plasmiogen (proactivator) Plasmin Thrombin Fibrinogen degradation products Lytic state Fibrinogen Fibrin Fibrin degradation products Clot dissolution Streptokinase Uses: PE, DVT, acute MI, arterial thrombosis, and occluded access shunts The greatest benefit of appears to be achieved by early administration (within 4 hrs) Since the advent of newer agents, streptokinase is rarely used clinically for fibrinolysis. It currently is not marketed in the U.S. Streptokinase- ADRs 1. Bleeding disorders: Aminocaproic acid inhibits streptokinaseplasminogen activator complex formation and can be used in the rare instances of lifethreatening hemorrhage Streptokinase-ADRs 2. Hypersensitivty (rash, fever, anaphylaxis) Foreign protein and is antigenic Circulating antibodies against streptokinase are likely to be present in most patients Esp. individuals who have had a streptococcal infections (antistreptococcal antibodies) Antibodies can combine with streptokinase and neutralize its fibrinolytic properties Anistreplase (anisoylated plasminogen streptokinase activator complex) Pro-drug consists of a complex of purified human plasminogen and bacterial streptokinase that has been acylated to protect the enzyme's active site When administered, the acyl group spontaneously hydrolyzes, freeing the activated streptokinase-plasminogen complex Anistreplase (anisoylated plasminogen streptokinase activator complex) Advatnages: 1. Rapid IV injection 2. Greater clot selectivity (ie, more activity on plasminogen associated with clots than on free plasminogen in the blood) 3. More thrombolytic activity