* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Drugs Used in Coagulation Disorders
Discovery and development of proton pump inhibitors wikipedia , lookup
Discovery and development of non-nucleoside reverse-transcriptase inhibitors wikipedia , lookup
Discovery and development of ACE inhibitors wikipedia , lookup
Clinical trial wikipedia , lookup
Drug discovery wikipedia , lookup
Pharmaceutical industry wikipedia , lookup
Pharmacokinetics wikipedia , lookup
Neuropharmacology wikipedia , lookup
Metalloprotease inhibitor wikipedia , lookup
Pharmacognosy wikipedia , lookup
Drug-eluting stent wikipedia , lookup
Prescription costs wikipedia , lookup
Discovery and development of integrase inhibitors wikipedia , lookup
Theralizumab wikipedia , lookup
Drug interaction wikipedia , lookup
Psychopharmacology wikipedia , lookup
Neuropsychopharmacology wikipedia , lookup
Pharmacogenomics wikipedia , lookup
Discovery and development of direct Xa inhibitors wikipedia , lookup
Discovery and development of direct thrombin inhibitors wikipedia , lookup
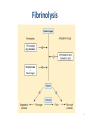
Drugs Used in Coagulation Disorders Presented by Dr. Sasan Zaeri PharmD, PhD Mechanism of blood coagulation 2 Mechanism of blood coagulation 3 Fibrinolysis 4 5 ANTICOAGULANTS Classification • Three major types of anticoagulants: – Heparin and related products • must be used parenterally – Direct thrombin inhibitors • used parenterally – Orally active coumarin derivatives (e.g. warfarin) 6 ANTICOAGULANTS Heparin • A large sulfated polysaccharide polymer obtained from animal sources • Highly acidic and can be neutralized by basic molecules – Protamine sulfate (heparin antidote) • Given IV or SC to avoid the risk of hematoma associated with IM injection 7 ANTICOAGULANTS Heparin • Low-molecular-weight (LMW) heparin – Enoxaparin, Dalteparin, Tinzaparin – Greater bioavailability (SC) – Longer durations of action • Administered once or twice a day • Fondaparinux – A small synthetic drug that contains the biologically active pentasaccharide – Administered SC once daily 8 Heparin Mechanism and effects • Heparin binds to antithrombin III (ATIII): – irreversible inactivation of thrombin and factor Xa • 1000-fold faster than ATIII alone • Heparin provides anticoagulation immediately after administration • Heparin monitoring – Activated partial thromboplastin time (aPTT) 9 Mechanism of blood coagulation 10 Mechanism and effects • LMW heparins and fondaparinux – bind ATIII – same inhibitory effect on factor Xa as heparin– ATIII – they fail to affect thrombin • a more selective action – aPTT not required • potential problem in renal failure due to decreased clearance 11 Clinical uses • When anticoagulation is needed immediately e.g. when starting therapy • Common uses: – DVT – Pulmonary embolism – acute myocardial infarction • in combination with thrombolytics for revascularization • in combination with glycoprotein IIb/IIIa inhibitors during angioplasty and placement of coronary stents • The drug of choice in pregnancy 12 Toxicity • Increased bleeding (most common) – may result in hemorrhagic stroke – Protamine as antidote • Not effective for LMW heparins and fondaparinux • Heparin-induced thrombocytopenia (HIT) • • • Due to antibody against complex of heparin and platelet factor 4 May yield venous thrombosis less likely with LMW heparins and fondaparinux • Osteoporosis – Due to prolonged use of unfractionated heparin 13 Direct Thrombin Inhibitors • Lepirudin – Recombinant form hirudin (Hirudo medicinalis) • Desirudin and Bivalirudin – Modified forms of hirudin • Argatroban – A small molecule with a short half-life • Dabigatran – Orally active 14 Mechanism and effects • These drugs inhibit both soluble thrombin and the thrombin enmeshed within developing clots • Bivalirudin – also inhibits platelet activation 15 Clinical uses • Alternatives to heparin – primarily in patients with HIT • Coronary angioplasty – Bivalirudin in combination with aspirin Monitoring using aPTT requiured 16 Toxicity • Bleeding – No reversal agents exist • Anaphylactic reactions – Prolonged infusion of lepirudin induces antibodies that form a complex with lepirudin and prolong its action 17 Warfarin • Small lipid-soluble molecule – readily absorbed after oral administration • Highly bound to plasma proteins (>99%) • Its elimination depends on metabolism by cytochrome P450 enzymes 18 Mechanism of action • Warfarin inhibits vitamin K epoxide reductase (VKOR) in liver – ↓ reduced form of vitamin K → ↓ factors II, VII, IX, X, protein C and S 19 • Anticoagulant effect is observed within 8-12 h • The action of warfarin can be reversed by: – Vitamin K1 (slowly within 6-24 h) – Transfusion with fresh or frozen plasma (more rapid reversal) • Warfarin monitoring: – Prothrombin time (PT) expressed by INR – INR: 2-3 20 Clinical uses • Chronic anticoagulation in all of the clinical situations described for heparin – Exception: anticoagulation in pregnant women • In DVT 1. Heparin + warfarin (5-7 days) 2. Warfarin (3-6 months) 21 Warfarin toxicity • Bleeding (most common) • Hypercoagulability early in therapy → dermal vascular necrosis – due to deficiency of protein C • Bone defects and hemorrhage in fetus – Contraindicated in pregnancy 22 Warfarin toxicity • Drug interactions – Cytochrome P450 inducers • carbamazepine, phenytoin, rifampin, barbiturates – Cytochrome P450 inhibitors • amiodarone, selective serotonin reuptake inhibitors, cimetidine 23 24 THROMBOLYTIC AGENTS • Streptokinase – synthesized by streptococci • Alteplase, Tenecteplase and Reteplase – Recombinant forms of t-PA 25 Mechanism of Action • Conversion of plasminogen to plasmin 26 Clinical Uses • Alternative to coronary angioplasty – Best result in ST-elevated MI and bundle branch block – Prompt recanalization if used within 6 h • Ischemic stroke – Better clinical outcome if used within 3 h – Cerebral hemorrhage must be ruled out before such use • Severe pulmonary embolism 27 Toxicity • Bleeding – Same frequency with all thrombolytics – Cerebral hemorrhage (most serious manifestation) • Allergic reactions (streptokinase) – Even at first dose (streptococcal infection history) – Loss of drug efficacy – Not observed with recombinant forms of t-PA • BUT, t-PA is more expensive and not much more effective 28 ANTIPLATELET DRUGS 29 ANTIPLATELET DRUGS • Aspirin acts on COX irreversibly – several-day effect • Other NSAIDs not used as antiplatelet drug – May interfere with aspirin antiplatelet effect • Abciximab (monoclonal antibody), eptifibatide and tirofiban – reversibly inhibit glycoprotein IIb/IIIa • Clopidogrel, ticlopidine – irreversibly inhibit the platelet ADP receptor 30 ANTIPLATELET DRUGS • Dipyridamole and cilostazol – Inhibit phosphodiesterase enzymes → ↑ cAMP and cGMP – Inhibit uptake of adenosine by endothelial cells and RBCs • Adenosine acts through platelet adenosine A2 receptors to increase platelet cAMP 31 Clinical Uses • Aspirin – To prevent first or further MI – To prevent transient ischemic attacks, ischemic stroke, and other thrombotic events 32 Clinical Uses • Glycoprotein IIb/IIIa inhibitors – To prevent restenosis after coronary angioplasty – In acute coronary syndromes (unstable angina and non-Qwave acute MI) • Clopidogrel and ticlopidine – To prevent transient ischemic attacks and ischemic strokes • especially in patients who cannot tolerate aspirin – To prevent thrombosis in patients with coronary artery stent (clopidogrel) 33 Clinical Use • Dipyridamole – To prevent thrombosis in those with cardiac valve replacement (adjunct to warfarin) – To treat intermittent claudication (a manifestation of peripheral arterial disease) 34