* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Document
Nucleic acid analogue wikipedia , lookup
Citric acid cycle wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Peptide synthesis wikipedia , lookup
Genetic code wikipedia , lookup
Butyric acid wikipedia , lookup
Proteolysis wikipedia , lookup
Glyceroneogenesis wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Biosynthesis wikipedia , lookup
Biochemistry wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
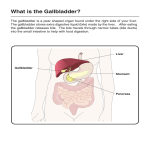
2016-11-08 Steroids: cholesterol, Bile acids and steroid hormones Vitamin D 1. Structure and stereochemistry of steroids 2. Cholesterol as a precursor of bile acids, steroid hormones and vitamin D3 3. Structure of the primary and secondary bile acids, conjugation with glycine and taurine 4. Steroid hormones - progestins, glucocorticoids, mineralocorticoids, androgens, estrogens 5. Vitamin D and its active forms Structure and stereochemistry of steroids Steroids are derivatives of perhydrocyclopentanophenanthrene system. the ring 1 2016-11-08 STEROID NUMBERING SYSTEM 18 11 19 1 2 A 3 10 17 C 13 D 14 9 B8 16 15 7 5 4 12 6 Steroid ring • The steroid ring is not planar, but flattened in a way that it extends as much as possible. • All the rings are in as much of a chair conformation as possible. • There is no rotation possible around bonds in the rings, which makes sterols pretty rigid, except for the chain at C17. Conformation • There are four rings in a steroid skeleton and hence there are three fusion points. A/B, B/C and C/D rings share two carbons each (fusion). 2 2016-11-08 An A-B cis steroid The methyl groups are attached at points of ring junction and are called angular methyl groups. Other groups on the same side of the steroid plane as the angular methyl groups are said to be β substitutents Groups below the plane of the steroid ring system are said to be α substituents The main sterol found in the membranes of eukaryotic cells is cholesterol. The polar head in this lipids is the hydroxyl in carbon C3. 3 2016-11-08 Cholesterol One of the most widely occuring steroids, was first isolated in 1770. Contains 8 chiral C atoms, this means that 28 or 256 stereoisomers are possible, but only one of them is cholesterol. Contains 27 carbons Cholesterol sources, biosynthesis and degradation • Slightly less than half of the cholesterol in the body derives from biosynthesis de novo. • Biosynthesis in the liver accounts for approximately 10%, and in the intestine approximately 15%, of the amount produced each day. • Cholesterol synthesis occurs in the cytoplasm and microsomes from the two-carbon acetate group of acetyl-CoA. Biosynthesis of Cholesterol The process has five major steps: • 1. Acetyl-CoAs are converted to 3-hydroxy-3-methylglutarylCoA (HMG-CoA) • 2. HMG-CoA is converted to mevalonate • 3. Mevalonate is converted to the isoprene based molecule, isopentenyl pyrophosphate (IPP), with the concomitant loss of CO2 • 4. IPP is converted to squalene • 5. Squalene is converted to cholesterol. 4 2016-11-08 Acetyl CoA is the source of all carbon atoms in cholesterol Acetyl CoA CoA Acetoacetyl CoA Acetyl CoA CoA β -hydroxy- β- methylglutaryl CoA HMG-CoA reductase Mevalonate Squalene Farmesyl pyrophosphate Cyclization HMG-CoA Reductase HMG-CoA reductase 1. integral membrane protein in the ER 2. carries out an irreversible reaction 3. is an important regulatory enzyme in cholesterol synthesis Fate of Cholesterol • Incorporated into hepatocyte membranes • Exported – Bile acids – Cholesterol esters – Free cholesterol 5 2016-11-08 Cholesterol Esters • Acyl-CoA:cholesterol acyl transferase (ACAT) is an ER membrane protein • ACAT transfers fatty acid of CoA to C3 hydroxyl group of cholesterol • Excess cholesterol is stored as cholesterol esters in cytosolic lipid droplets Fig. 8 ROLES OF CHOLESTEROL AND BILE ACIDS (SALTS) The physiological roles of cholesterol include: a) b) c) an important lipid component of biological membranes, precursor of steroid hormones and source of bile acids. Bile acids are polar derivatives of cholesterol and aid in: a) b) c) lipid digestion lipid absorption cholesterol excretion Bile Acids Synthesis and Utilization • The end products of cholesterol utilization are the bile acids, synthesized in the liver. • Synthesis of bile acids is one of the predominant mechanisms for the excretion of excess cholesterol. • However, the excretion of cholesterol in the form of bile acids is insufficient to compensate for an excess dietary intake of cholesterol. 6 2016-11-08 Bile • • • Bile is formed in the liver and secreted down the bile duct into the gallbladder, from where it passes into the intestine The gallbladder stores bile produced by the liver between meals. During digestion, the gallbladder contracts and supplies bile rapidly to the duodenum by way of the common bile duct Bile • Bile is a complex solution of salts and protein, containing micelles composed of cholesterol, phospholipids and bile salts. • The composition of these micelles is critical, as imbalance may lead to crystalization of cholesterol in the gallbladder, leading to the formation of gallstones. GALLSTONES • Cholesterol gallstones can form as a result of excessive secretion of cholesterol or of insufficient amounts of bile acids and lecithin relative to cholesterol in bile • If the amount of cholesterol in bile acids get above 15%, then gallstones may result. • If cholesterol gets too high, then they may crystallize out of solution in the gall bladder, where bile is concentrated tenfold before secretion. 7 2016-11-08 Functions of Bile Salts Important for cholesterol excretion: 1. As metabolic products of cholesterol 2. Solubilizer of cholesterol in bile Emulsifying factors for dietary lipids, a prerequisite step for efficient lipid digestion Cofactor for pancreatic lipase and PLA2 Facilitate intestinal lipid formation of mixed micelle absorption by Bile salts • • • detergent character of bile salts is due to the hydrophobic-hydrophilic nature of the molecules the presence of hydroxyl (or sulfate) and the terminal carboxyl group on the tail gives the molecule its hydrophilic face the steroid ring with its puckered plane provides the hydrophobic face Emulsification of Dietary Lipids in Duodenum: Role of Bile Salts • Emulsification increases the surface area of lipid droplets, therefore the digestive enzymes can effectively act. • Mechanisms: 1. Mechanical mixing by peristalsis 2. Detergent effect of bile salts: Bile salts interact with lipid particles and aqueous duodenal contents, stabilizing the particles as they become smaller, and preventing them from coalescing. 8 2016-11-08 Absorption of Lipids by Intestinal Mucosal Cells: Role of Bile salts Mixed micelles: Disc-shaped clusters of amphipathic lipids. Arranged with their hydrophobic groups on the inside and their hydrophilic groups on the outside. Micelle includes end products of lipid digestion, bile salts and fat-soluble vitamins Note: Short- and medium-chain fatty acids do not require mixed micelle for absorption by intestinal cells The bile acids are amphipathic, with detergent properties. They emulsify fat globules into smaller micelles, increasing the surface area accessible to lipid-hydrolyzing enzymes. Synthesis of Bile Salts Fig. 9 • • • Fig. 10 Rate-limiting step performed by the 7α-hydroxylase (CYP7A1) and is regulated by bile salt concentration End product: Cholic acid series & Chenocholic acid series Bile salts can be conjugated & become better detergents 9 2016-11-08 Synthesis of bile salts Primary bile acids Bile Salts • • • • Bile acids & salts are effective detergents Synthesized in the liver Stored & concentrated in the gallbladder Discharged into gut and aides in absorption of intraluminal lipids, cholesterol, & fat soluble vitamines • Bile acid refers to the protonated form while bile salts refers to the ionized form – The pH of the intestine is 7 and the pKa of bile salts is 6, which means that 50% are protonated • These terms are sometimes used interchangeably SYNTHESIS OF BILE SALTS • Bile salts are just bile acids with the carboxylate function modified by adding an amine to it via an amide linkage. • Bile Salts are even more polar than their corresponding acids. • Glycocholate: Cholate with glycine added as an amide function. • Taurocholate: Cholate with Taurine added as an amide function. Taurine is a modified cysteine. 10 2016-11-08 Primary and secondary bile acids • Chenodeoxycholic acid (45%) and cholic acid (31%) are referred to as the primary bile acids. • Within the intestines the primary bile acids are acted upon by bacteria and converted to the secondary bile acids, identified as deoxycholate (from cholate) and lithocholate (from chenodeoxycholate). • Both primary and secondary bile acids are reabsorbed by the intestines and delivered back to the liver via the portal circulation. Types of Bile Acids/Salts • Primary bile acids – Good emulsifying agents • All OH groups on same side • pKa = 6 (partially ionized) • Conjugated bile salts – Amide bonds with glycine or taurine – Very good emulsifier • pKa lower than bile acids 11 2016-11-08 Primary Bile Acids and Salts Cholic acid BILE ACIDS Glycocholic Taurocholic BILE SALTS Chenodeoxycholic acid Glycochenodeoxycholic Taurochenodeoxycholic Bile salts (Conjugated bile acids): amide-linked with glycine or taurine The ratio of glycine to taurine forms in the bile is 3:1 Bile acids are excreted in conjugated form, as taurocholic acid and glycocholic acid Bile acids are conjugated with glycine or taurine before being secreted into bile, where the ratio of glycine to taurine-conjugated acid is about 3:1 Fate of the bile salts and the synthesis of secondary bile acids Intestinal bacteria deconjugate and dehydroxylate the bile salts removing the glycine and taurine residues and the hydroxyl group at position 7. The secondary bile salts are less soluble and less readily resorbed from the intestinal lumen. Lithocholic acid has a hydroxyl group only at position 3, is the least soluble bile acid, its major fate is excretion. 12 2016-11-08 Secondary Bile Acids Bile salts Glyco- or Tauro-cholate Glycine Taurine Bile acids -Chenodeoxycholate Intestinal bacteria Cholic acid Chenodeoxycholic Intestinal bacteria OH 2° Bile acids Deoxycholic acid Lithocholic Secondary bile acids Primary bile acids •Within the intestines the primary bile acids are converted by bacteria into the secondary bile acids, identified as deoxycholate (from cholate) and lithocholate (from chenodeoxycholate). Steroids Grups –OH localized at: C-3 C-7 C-12 cholic acid OH OH OH chenodeoxycholic acid OH OH H deoxycholic acid OH H OH lithocholic acid OH H H Primary bile acids: Secondary bile acids: 13 2016-11-08 The pK of the bile acids is about 6. Therefore, in the contents of the intestinal lumen, which normally have a pH of 6, about half of the molecules are present in the protonated form and half are ionized, forming bile salts. The carbonyl group at the end of the side chain is activated by a reaction that requires ATP and coenzyme A. The acyl CoA derivatives can react with either glycine or taurine, forming amides that are known as the conjugated bile salts. The bile acids conjugated with glycine have a pK of about 4, so a higher percentage of the molecules is present in the ionized form at the pH of the intestine. The taurine conjugates have a pK of about 2, and even greater percentage of the molecules of these conjugates are ionized in the lumen of the gut. Taurine is synthesized from the amino acid cysteine. Clinical Significance of Bile Acid Synthesis • Bile acids perform four physiologically significant functions: • 1. Their synthesis and subsequent excretion in the feces represent the only significant mechanism for the elimination of excess cholesterol. • 2. Bile acids and phospholipids solubilize cholesterol in the bile, thereby preventing the precipitation of cholesterol in the gallbladder. • 3. They facilitate the digestion of dietary triacylglycerols by acting as emulsifying agents that render fats accessible to pancreatic lipases. • 4. They facilitate the intestinal absorption of fat-soluble vitamins. •the primary and secondary bile acids are reabsorbed almost exclusively in the ileum returning to the liver by way of the portal circulation (98 to 99%) •the entherohepatic circulation 14 2016-11-08 Recycling of bile salts Bile salts are synthesized in the liver, stored in the gallbladder, secreted into the small intestine, resorbed in the ileum, and returned to the liver. Only 5% are excreted. Cholesterol An amphipathic lipid, an essential structural component of membranes and the outher layer of plasma lipoproteins, Present in tissues and lipoproteins either as a free cholesterol or cholesteryl ester, Lipoproteins transport free cholesterol in the circulation, where it readily equilibrates with cholesterol in other lipoproteins and in membranes, Cholesteryl ester is a storage form of cholesterol in most tissues, Low density lipoprotein (LDL) is the mediator of cholesterol and cholesteryl ester uptake into many tissues, Free cholesterol is removed from tissues by high density lipoprotein (HDL) and transported to the liver for conversion to bile acids, Excess cholesterol is excreted from the liver in the bile as cholesterol or bile salts; a large proportion of bile salts is absorbed into the portal vein and returned to the liver as part of the entherohepatic circulation, Elevated levels of cholesterol are associated with atherosclerosis Cholesterol in the cell membrane Cholesterol is also present in the membrane. It maintains the fluidity and increases the stability of the membrane. Without cholesterol the membrane would easily split apart 15 2016-11-08 The Utilization of Cholesterol • • • Cholesterol is transported in the plasma predominantly as cholesteryl esters associated with lipoproteins. Dietary cholesterol is transported from the small intestine to the liver within chylomicrons. Cholesterol synthesized by the liver, as well as any dietary cholesterol in the liver that exceeds hepatic needs, is transported in the serum within LDLs (Low density lipoproteins). The Acyl-Co: cholesterol acyltransferase (ACAT) reaction (Synthesis of Cholesterol Esters by ACAT) Located mainly in the endoplasmic reticulum ACAT catalyzes the covalent joining of cholesterol and long-chain fatty acyl-CoA to form cholesterol esters. Cholesterol ester products of the ACAT reaction are either stored in cytosolic droplets or secreted from cells as components of apo-B lipoproteins. HDL High-density lipoprotein (HDL) begins in the liver and small intestine as small protein rich particles containing relatively little cholesterol and cholesterol ester HDLs contain apoA-I and apoA-II, as well as the enzyme lecithin-cholesterol acyltransferase (LCAT) which catalyzes the formation of cholesterol ester 16 2016-11-08 Functions of HDL • • • • Transfers proteins to other lipoproteins Picks up lipids from other lipoproteins Picks up cholesterol from cell membranes Converts cholesterol to cholesterol esters via the LCAT reaction • Transfers cholesterol esters to other lipoproteins, which transport them to the liver (referred to as “reverse cholesterol transport) Reverse Cholesterol Transport • An enzyme present in our circulation, Lecithin Cholesterol Acyl Transferase (LCAT), removes one fatty acid from Phosphatidylcholine (PC) and attaches it to cholesterol, converting cholesterol into a cholesterol ester, which is then taken up by a HDL lipoprotein and transported back to the liver, from where the cholesterol is excreted from the body in the form of a bile salt Acyltransferase lecithin : cholesterol (LCAT) The reaction catalyzed by LCAT. This enzyme is present on the surface of HDL, and stimulated by the HDL component of apoA-1. 17 2016-11-08 Lipoproteins and Lipid Transport Steroid hormones Steroid hormones are grouped into five categories: - progestins, - glucocorticoids, - mineralocorticoids, - androgens, - estrogens. All contain the four-ring structure of the steroid nucleus and are remarkably similar in structure, considering the enormous differences in their physiological effects. They mediate a wide variety of vital physiological functions. FUNCTIONS: Steroids Hormones • Cholesterol: precursor of the five major classes of steroid hormones • Glucocorticoid : carbohydrate metabolism • Mineralocorticoid: Sodium retention in renal tubules • Androgen, Estrogen, Progestagens: reproductive system 18 2016-11-08 Steroid hormones The primary organs involved in their biosynthesis are the gonads and the adrenal cortex, and the placenta in pregnant females. In steroid hormones, the side chain of cholesterol is either shortened or nonexistent. Steroid hormones (SH) are not stored for release after synthesis. The level of circulating SH is controlled by its rate of synthesis, which is controlled by signals from the brain. Activation of SH synthesis involves stimulation of both hydrolysis of cholesterol esters and uptake cholesterol into mitochondria of cells in the target organ. SYNTHESIS OF STEROID HORMONES CHOLESTEROL ------> PREGNENOLONE • Removal of 6 of the side-chain carbons. This step is common to all steroid hormone synthesis. • • CHOLESTEROL ------> PREGNENOLONE This step occurs on the mitochondrial membrane. This leaves a C21 compound, pregnenolone, which may accurately be described as the mother of all steroids (the C21 steroid carbon skeleton is given the name pregnane). Steroids There is an enzyme complex called cholesterol desmolase that hydroxylates C-20 and C-22 and cleaves it to yield pregnolone Desmolase (in mitochondria) forms pregnenolone, precursor to all others 19 2016-11-08 activated to turn on pathways Cholesterol Progesterone Pregnenolone 11-Deoxycortisone Progesterone Aldosterone 21-hydroxylase 11-Deoxycortisol Progesterone 21-hydroxylase Cortisol General pathways for the synthesis of aldosterone and cortisol in the adrenal cortex Pregnenolone Cholesterol Progesterone Progesterone Testosterone (pathway ends here in testes) Androstenedione Estrone (produced in both male and female adipose cells) Estradiol (pathway continues to here in ovaries) In obese men, overproduction of estrogen in fat cells can cause gynecomastia = excessive male breast development Pathways for the synthesis of testosterone (testes) and the estrogens estradiol (ovaries) and estrone (adipose cells) Transformations of Cholesterol: Steroid Hormones OH O CH3 O HO OH CH3 O HO O Cortisol Cholesterol Progesterone OH OH OH O Testosterone HO CH 2 Estradiol HO OH Vitamin D 20 2016-11-08 Groups of steroid hormones 1. Progestins – regulate events during pregnancy, progesterone is the major hormone involved in sustaining pregnancy 2. Glucocorticoids –affect basal metabolism, host defense mechanisms, blood pressure, response to stress, promote gluconeogenesis and supress inflammation reactions (cortisol, corticosterone) 3. Mineralocorticoids – affect electrolyte balance and ion transport, regulate ion balance by promoting reabsorption in the kidney of Na+ , Cl-, HCO3- (aldosterone) 4. Androgens – promote male sexual differentiation, spermatogenesis, and maintain male characteristics (androstenedione, testosterone), 5. Estrogens – female sex hormones, stimulate the development of tissues involved in reproduction, support femal characteristics (estrone, estradiol) Table 1. Functions of Hormones Derived from Cholesterol Product Functions Progesterone prepares uterus lining for implantation of ovum Glucocorticoids (cortisol) (produced in adrenal cortex) (catabolic steroid) promote gluconeogenesis; favor breakdown of fat and protein (fuel mobilization); anti-inflammatory Mineralocorticoids (aldosterone) (produced in adrenal glands) maintains blood volume and blood pressure by increasing sodium reabsorption by kidney 21 2016-11-08 Table 1. Functions of Hormones Derived from Cholesterol Product Functions Androgens (strongest = testosterone) (produced in testes primarily but weak androgens in adrenal cortex) (anabolic steroid) development of male secondary sex characteristics; prevents bone resorption Estrogen development of female (produced in ovaries primarily but also secondary sex in adipose cells of males and females) characteristics; prevents bone resorption Vitamin D (not a steroid hormone) (produced in the skin in response to UV light and processed to active form in kidney) intestinal calcium absorption; promotes bone formation; prevents phosphate loss by kidneys Steroid hormones act in the nucleus to change gene expression Steroid hormones are too hydrophobic to dissolve readily in the blood, are carried on specific carrier proteins from the point of their release to their target tissues In the target tissue, these hormones pass through the plasma membrane by simple diffusion and bind to specific receptor proteins in the nucleus The hormone-receptor complexes act by binding to highly specific DNA sequences and altering gene expression 22 2016-11-08 Vitamin D • Vitamin D is a steroid prohormone, by various metabolic changes in the body it gives rise to a hormone known as calcitriol, which play a central role in calcium and phosphate metabolism. Formation of provitamin (7-dehydrocholesterol) D D Vitamins are generated from the provitamins ergosterol and 7dehydrocholesterol by the action of sunlight. Ergosterol occurs in plants and 7-dehydrocholesterol in animals. UV light from sunlight cleaves the B ring of both compounds. Ergocalciferol (vitamin D2) is formed in plants. Cholecalciferol (vitamin D3) is formed in exposed skin. A specific transport protein called the vitamin D-binding protein binds vitamin D3 and moves its from the skin or intestine to the liver, where it undergoes 25-hydroxylation 23 2016-11-08 Liver Diet OH 25hydroxylase HO HO 25(OH) D3 Vitamin D3 UV from sunlight Kidney 1-hydroxylase Skin OH HO Specific receptors in intestine, bone, kidney Ca: Intestinal absorption Renal reabsorption 7 Provitamin D3 (7-dehydrocholesterol: Intermediate in cholesterol synthesis) HO OH 1,25(OH)2 D3 PO4: Intestinal absorption Renal reabsorption (active hormone form) Figure 7. Photobiosynthesis of vitamin D3 and its metabolism 1,25-dihydroxycholecalcyferol (active form of D3) • Active VITAMIN D3 – B-Ring opens – 1-Hydroxylation – 25-Hydroxylation The product (1α,25 dihydroxyD3) is the most potent vitamin D metabolite. Its production is regulated by its own concentration, parathyroid hormone, and serum phosphate. vitamin D-deficiency diseases Deficiency of vitamin D causes rickets and osteomalacia. 24