* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download anaerobic respiration
Nitrogen cycle wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Cyanobacteria wikipedia , lookup
Biochemistry wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Sulfur cycle wikipedia , lookup
Magnetotactic bacteria wikipedia , lookup
Metalloprotein wikipedia , lookup
Citric acid cycle wikipedia , lookup
Electron transport chain wikipedia , lookup
Photosynthesis wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
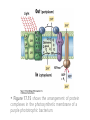
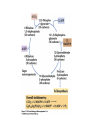
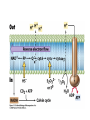
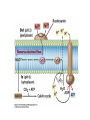
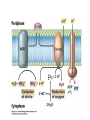
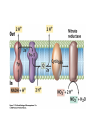
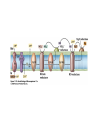
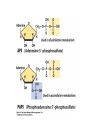
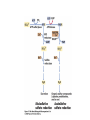
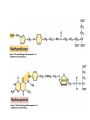
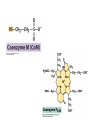
• Figure 17.10a shows a typical phycobilin, and Figure 7.10b shows the structure of a phycobilisome. • The absorption spectrum of a cyanobacterium that has a phycobiliprotein (phycocyanin) as an accessory pigment is shown in Figure 17.11. 17.4 Anoxygenic Photosynthesis, • In anoxygenic photosynthesis, a series of electron transport reactions in the photosynthetic reaction center of anoxygenic phototrophs results in the formation of a proton motive force and the synthesis of ATP. • The production of ATP in photosynthesis is called photophosphorylation. • Reducing power for CO2 fixation comes from reductants in the environment and requires reverse electron transport in purple phototrophs. Figure 17.13 illustrates the structure of the reaction center of purple phototrophic bacteria. • A general scheme of electron flow in anoxygenic photosynthesis in a purple bacterium is shown in Figure 17.14. • Figure 17.15 shows the arrangement of protein complexes in the photosynthetic membrane of a purple phototrophic bacterium. Photosynthesis in Other Anoxygenic Phototrophs • Figure 17.18 contrasts the photosynthetic electron flow of purple and green bacteria and the heliobacteria. 17.2 Concept Check A series of electron transport reactions occur in the photosynthetic reaction center of anoxygenic phototrophs, resulting in the formation of a proton motive force and the synthesis of ATP. Reducing power for CO2 fixation comes from reductants present in the environment and requires reverse electron transport in purple phototrophs. How does photophosphorylation compare with electron transport phosphorylation in respiration? What is reverse electron flow and why is it necessary? Which phototrophs need to use reverse electron flow? 17.3 Oxygenic Photosynthesis 17.3 Concept Check In oxygenic photosynthesis, water donates electrons to drive autotrophy, and oxygen is produced as a by-product. Two separate light reactions are involved, photosystems I and II. Photosystem I resembles the system in anoxygenic photosynthesis. Photosystem II is responsible for splitting H2O to yield O2. Why is the term noncyclic electron flow used in reference to oxygenic photosynthesis? What are the major differences between the two reaction center chlorophyll molecules in photosystems I and II regarding absorption properties and E0'? 17.4 Autotrophic CO2 Fixation: The Calvin Cycle RubisCO and the Formation of PGA Stoichiometry of the Calvin Cycle 17.4 Concept Check The fixation of CO2 by most phototrophic and other autotrophic organisms occurs via the Calvin cycle, in which the enzyme ribulose bisphosphate carboxylase (RubisCO) plays a key role. The Calvin cycle is an energy-demanding process in which CO2 is converted into sugar. What reaction does the enzyme ribulose bisphosphate carboxylase carry out? Why is reducing power needed for autotrophic growth? What is a carboxysome? 17.5 Autotrophic CO2 Fixation: Reverse Citric Acid Cycle and the Hydroxypropionate Cycle Carbon Dioxide Fixation * Calvin Cycle + . CO2 Fixation within many autotrophic microbial and plant cells . 3 CO2 + 9 ATP + 6 NADPH -> glyceraldehyde-3-phosphate + 9 ADP 6 NADP+ . Ribulose-1,5-bisphosphate carboxylase (RuBisCo) Caraboxysomes * Reductive Tricarboxylic Acid Cycle Pathway . Some potoautotrophs, such as the green sulfur bacterium Chlorobium, fix CO2 via a reverse (reductive) tricarboxylic acid cycle. . This is achieved at the expense of three ATP molecules. . The normal enzymes of the TCA cycle work in reverse of the normal oxidative direction of the cycle. . One exception ; Citrate lyase Citrate synthase Autotrophy in Chloroflexus * Hydroxypropionate Pathway . In the green nonsulfur bacterium, Chloroflexus, Two CO2 molecules are fixed and converted into one acetyl-CoA via the hydroxypropionate pathway. . The net result is that three CO2 molecules are converted into one pyruvic acid molecule. * C4 Pathway . The oxaloacetate formed in this pathway can then be used in amino acid and nucleic acid biosynthesis. . Although all organisms fix CO2 as part of their metabolism, heterotrophic organisms are unable to form a significant portion of their macromolecules from the C4 pathway alone. Assimilation of Organic C-1 Compounds * Methanotrophy . Bacteria that have the ability to use methane (CH4)- the most reduced form of carbon- as their sole carbon souce are called methanotrophs. . All methanotrophs are obligate aerobes that require O2 ; they are obligate C-1 utilizers. . Some methanotrophs such as Methylomonas, Methylococcus, and Methylosinus can grow on various C-1 compounds -methanol, for example-rather than only methane. . Methane monooxygenase . wide range substrate specificity . oxidation of ammonium ions, chloromethane, bromoethane, ethane, propane, trichloroethylene, and various other compounds . CH4 + O2 + NADH ---> CH3OH + H2O + NAD+ CH4 + O2 + Cytochrome C (reduced) ---> CH3OH + H2O + Cytochrome C (reduced) . The methanol formed by methane monooxygenase is further oxidized to formaldehyde. . Type 1 methanotrophs (Methylomonas, Methylococcus) ; Ribulose monophosphate cycle . Type II methanotrophs (Methylosinus) ; Serine pathway * Methylotrophy . The more general class of heterotrophic aerobes that can utilize onecarbon organic molecules other than methane, are called methylotrophs. . Some Pseudomonas. Bacillus, and Vibrio species use methanol, formate, or methylamine as a carbon source. . The methylotrophs use the serine pathway for assimilating C-1 compounds into organic molecules. 17.5 Concept Check The reverse citric acid cycle and the hydroxypropionate cycle are pathways of CO2 fixation found in green sulfur and green nonsulfur bacteria, respectively. Including the route of CO2 fixation, discuss at least three ways that you could distinguish a purple sulfur bacterium from a green sulfur bacterium. Including the route of CO2 fixation, what similarities and differences exist between green sulfur and green nonsulfur bacteria? II CHEMOLITHOTROPHY 17.8 Inorganic Electron Donors and Energetics 17.8 Concept Check Chemolithotrophs are able to oxidize inorganic chemicals as their sole sources of energy and reducing power. Most chemolithotrophs are also able to grow autotrophically. For what two purposes are inorganic compounds used by chemolithotrophs? Why does the oxidation of H2 yield more energy with O2 as electron acceptor than with SO42– as electron acceptor? 17.9 Hydrogen Oxidation • The hydrogen bacteria can oxidize H2 compounds, thereby generating a proton motive force and ATP synthesis (Figure 17.25). These chemolithotrophs are also autotrophs and fix CO2 via the Calvin cycle. 17.10 Oxidation of Reduced Sulfur Compounds • The sulfur bacteria can oxidize reduced sulfur compounds such as H2S and S0 (Figure 17.27). These chemolithotrophs are also autotrophs and use the Calvin cycle to fix CO2. 17.9–17.10 Concept Check Hydrogen (H2) and reduced sulfur compounds such as H2S and S0 are excellent electron donors for chemolithotrophs. These compounds can be oxidized by the hydrogen bacteria or the sulfur bacteria, respectively, thereby generating a proton motive force and ATP synthesis. These chemolithotrophs are also autotrophs and fix CO2 by the Calvin cycle. What special enzyme is needed for growth on H2? How many electrons are available from the oxidation of H2S if S0 is the final product? If SO42– is the final product? 17.11 Iron Oxidation • The iron bacteria are chemolithotrophs that use ferrous iron (Fe2+) as their sole energy source (Figure 17.30). • Most iron bacteria grow only at acid pH and are often associated with acid pollution from mineral and coal mining. Some phototrophic purple bacteria can oxidize Fe2+ to Fe3+ anaerobically. 17.11 Concept Check The iron bacteria are chemolithotrophs able to use ferrous iron (Fe2+) as sole energy source. Most iron bacteria grow only at acid pH and are often associated with acid pollution from mineral and coal mining. Some phototrophic purple bacteria can oxidize Fe2+ to Fe3+ anaerobically. Why is only a very small amount of energy available from the oxidation of Fe2+ to Fe3+ at acidic pH? What is the function of rusticyanin and where is it found in the cell? How can Fe2+ be oxidized anoxically? 17.12 Nitrification and Anammox • In anoxic ammonia oxidation (anammox), the nitrifying bacteria can use ammonia and nitrite as electron donors, a process called nitrification. • The ammonia-oxidizing bacteria produce nitrite (Figure 17.32), which is then oxidized by the nitrite-oxidizing bacteria to nitrate (Figure 17.33). Anoxic NH3 oxidation is coupled to both N2 and NO3– production in the anammoxosome. 17.12 Concept Check Ammonia and nitrite can be used as electron donors by the nitrifying bacteria. The ammonia-oxidizing bacteria produce nitrite, which is then oxidized by the nitrite-oxidizing bacteria to nitrate. Anoxic NH3 oxidation is coupled to both N2 and NO3– production in the anammoxosome. What is the inorganic electron donor for Nitrosomonas? For Nitrobacter? What are the substrates for the enzyme ammonia monooxygenase? What do nitrifying bacteria use as a carbon source? What is the anammox reaction and how does it differ from aerobic nitrification? III THE ANAEROBIC WAY OF LIFE: ANAEROBIC RESPIRATIONS 17.13 Anaerobic Respiration Alternative Electron Acceptors and the Electron Tower • Although oxygen is the most widely used electron acceptor in energy-yielding metabolism, a number of other compounds can be used as electron acceptors. •This process of anaerobic respiration is less energy efficient but enables respiration in environments where oxygen is absent. • Examples of anaerobic respiration are illustrated in Figure 17.35. Assimilative and Dissimilative Metabolism 17.13 Concept Check Although oxygen is the most widely used electron acceptor in energy-yielding metabolism, a number of other compounds can be used as electron acceptors. This process of anaerobic respiration is less energy efficient but makes it possible for respiration to occur in environments where oxygen is absent. What is anaerobic respiration? With H2 as electron donor, why is the reduction of NO3– a more favorable reaction than the reduction of S0? 17.14 Nitrate Reduction and the Denitrification Process • Nitrate is commonly used as an electron acceptor in anaerobic respiration. Its use requires the enzyme nitrate reductase, which reduces nitrate to nitrite. Many bacteria that use nitrate in anaerobic respiration eventually produce N2, a process called denitrification. • Figure 17.36 shows steps in the dissimilative reduction of nitrate. Biochemistry of Dissimilative Nitrate Reduction • Figure 17.37 shows electron transport processes in the membrane of Escherichia coli when O2 or NO3– is used as an electron acceptor and NADH is the electron donor. Other Properties of Denitrifying Prokaryotes 17.14 Concept Check Nitrate is a commonly used electron acceptor in anaerobic respiration. Its use requires the enzyme nitrate reductase that reduces nitrate to nitrite. Many bacteria that use nitrate in anaerobic respiration eventually produce N2, a process called denitrification. For Escherichia coli, why is more energy released in aerobic respiration than during NO3– reduction? Where is the dissimilative nitrate reductase found in the cell? What unusual metal does it contain? Why does an organism like Pseudomonas stutzeri derive more energy from NO3– respiration than does Escherichia coli? 17.15 Sulfate Reduction • The sulfate-reducing bacteria reduce sulfate to hydrogen sulfide. A summary of the oxidation states of the key sulfur compounds is given in Table 17.3. Biochemistry and Energetics of Sulfate Reduction • The reduction of sulfate first requires activation by a reaction with ATP to form the compound adenosine phosphosulfate (APS) (Figure 17.38). • Electron donors for sulfate reduction include organic compounds, H2, and even phosphite (HPO3–). • Disproportionation of sulfur compounds is an additional energy-yielding strategy for certain members of this group. • Figure 17.39 illustrates electron transport and energy conservation in sulfate-reducing bacteria. Acetate Use and Autotrophy Sulfur Disproportionation Phosphite Oxidation 17.15 Concept Check The sulfate-reducing bacteria reduce sulfate to hydrogen sulfide. The reduction of sulfate first requires activation by a reaction with ATP to form the compound adenosine phosphosulfate (APS). Electron donors for sulfate reduction include organic compounds, and even phosphite. Disproportionation of sulfur compounds is an additional energy-yielding strategy for certain members of this group. Identify the following: S0, SO42–, SO32–, S2O32–, H2S. How is sulfate converted to sulfite during dissimilative sulfate reduction? Why is H2 of importance to sulfate-reducing bacteria? Give an example of disproportionation. 17.16 Acetogenesis • In homoacetogenesis, anaerobes reduce CO2 to acetate, usually with H2 as the electron donor. An overview of the processes of methanogenesis and acetogenesis is shown in Figure 17.40. Organisms and Pathway Reactions of the Acetyl-CoA Pathway • The mechanism of acetate formation is the acetyl-CoA pathway (Figure 17.41), a series of reactions widely distributed in obligate anaerobes as either a mechanism of autotrophy or for acetate 17.16 Concept Check Homoacetogens are anaerobes that reduce CO2 to acetate, usually with H2 as electron donor. The mechanism of acetate formation is the acetyl-CoA pathway, a series of reactions widely distributed in obligate anaerobes as either a mechanism of autotrophy or for acetate catabolism. Draw the structure of acetate and identify the carbonyl group and the methyl group. What key enzyme of the acetyl-CoA pathway produces the carbonyl group of acetate? How do homoacetogens make ATP from the synthesis of acetate? If catabolism of fructose via glycolysis yields only two molecules of acetate, how can Clostridium aceticum ferment fructose by this pathway and produce three molecules of acetate? 17.17 Methanogenesis C1 Carriers in Methanogenesis Redox Coenzymes Biochemistry of CO2 Reduction to CH4 Methanogenesis from Methyl Compounds and Acetate