* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Hemoglobin and Cytochrome c
G protein–coupled receptor wikipedia , lookup
Clinical neurochemistry wikipedia , lookup
Interactome wikipedia , lookup
Silencer (genetics) wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Oxidative phosphorylation wikipedia , lookup
Drug design wikipedia , lookup
Western blot wikipedia , lookup
Signal transduction wikipedia , lookup
Ligand binding assay wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
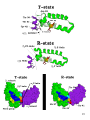
Amino acid synthesis wikipedia , lookup
Genetic code wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Biosynthesis wikipedia , lookup
Proteolysis wikipedia , lookup
Point mutation wikipedia , lookup
The relationship between structure and function is a central concept in Biochemistry. The book emphasizes myoglobin and hemoglobin to illustrate the ideas These proteins bind and release O 2. Hb also removes CO 2. . Mb - single polypeptide chain folded about a prosthetic group “heme”. Hb is a tetramer of the form α 2β 2 with two equivalent subunits. Mb: 153 amino acids MW ~ 17kD Secondary structure 77% α-- helix, 23% random coil. 121 residues in helical form with lengths running from 7 26 residues. 1 Helical segments A H fold back on each other to form a globular cavity for the heme group. Proteins without prosthetic groups cannot bind O 2 . But transition metals such as iron (Fe II) and Copper (Cu II) can bind O 2 . 2 Myoglobin Heme 3 4 Hemoglobin (Hb) functions to transport oxygen in the blood of all warm-blooded animals. It is a multimeric protein consisting of four subunits, alpha1 , beta1, alpha2 and beta2. All four subunits resemble both themselves and myoglobin. The subunits are symmetrically arranged. Note the extensive contacts at between alpha and beta subunits, while there is little alpha-alpha and beta-beta contact (as they face each other across a 20 Å channel that runs through the center of the protein.) Also note the heme group located within a deep cleft in the side of each of the subunits. The heme groups are both the binding site for oxygen and the starting point for the mechanism of oxygen binding cooperativity. Above is a diagram of the alpha1 subunit of Hb. Oxygen binds to the Fe(II) atom, directly below the plane of the heme group and opposite the proximal histidine. Note how the top and bottom of the heme group are surrounded by the protein globule. This prevents two Hb heme groups from binding to the same oxygen molecule, which would allow one heme group to catalyze the autooxidation Fe(II) to Fe(III) in the other, resulting in irreversable oxygen binding. 5 Hemoglobin: Structure & Function Hemoglobin is a large protein (66.7 kD) coupled to four porphyrins or heme moities.The globin portion of hemoglobin consists of four polypeptide chains ( “a” with 141aa and “ß” with 146aa )arranged in pairs forming a tetramer. Each globin chain is covalently attached to a heme moiety. The bonds between a and ß chains are weaker than between similar globin chains, forming a natural cleavage plane, the a1ß2 interface, important for oxygen binding and release. When this cleavage is open [R (relaxed) state] oxygen can bind (high oxygen affinity). When the two a1ß2 interfaces are closely bound [T (taut) state] the Hgb molecule has a low affinity for oxygen. 6 The globin fold is found in many related proteins such as myoglobin and hemoglobin. Usually, it has eight a-helices, designated AH. These eight helices pack into two layers, making an angle of about 50o between them. In each layer, these a-helices are arranged in antiparallel fashion into a pattern called a Greek key helix bundle. Specifically, the sperm whale myoglobin has 153 aa residues and a heme group. The eight helices in myoglobin have the following aa segments: Helix A: SER3 -- GLU18 B: ASP20 -- SER35 C: HIS36 -- LYS42 D: THR51 -- ALA57 E: SER58 -- LYS77 F: LEU86 -- THR95 G: PRO100 -- ARG118 H: LY124 -- LEU149 7 The heme (Fe(II)-protoporphyrin IX) is the prosthetic group of myoglobin as well as hemoglobin; it is the oxygen binding site. Structurally, the heme is an iron-coordinated porphyrin, a macro cyclic ring system composed of four pyrroles with four methyl, two vinyl, and two propionic acid side chains. All atoms in the tetrapyrrole ring (neglecting the side chains) lie nearly in the same plane. 8 9 The Fe atom is chelated by a conjugated tetrapyrrole ring system - Protoporphyrin IX. The protoporphyrin IX-Fe II complex is called heme.The visible absorption spectrum of heme is responsible for the color of blood. Heme is concovalently bound to globin in a hydrophobic crevice in both Mb and Hb. 10 Each heme group can bind one oxygen molecule. Thus, the hemoglobin tetramer can bind a total of four oxygen molecules. Hemoglobin also serves the purpose of transporting carbon dioxide. Oxygen is bound by hemoglobin at the lungs, carried through the blood stream to various tissues, where it gives up the oxygen, and binds carbon dioxide and transports it back to the lungs. Carbon dioxide is bound as the bicarbonate ion, HCO3-, at the dimer interface and not to the heme. The binding of oxygen to hemoglobin is cooperative. Thus, binding of one oxygen causes the next oxygen to bind more strongly, the third oxygen even more strongly and so on. In fact, hemoglobin is usually observed in only two states - all four chains carrying oxygen, or completely oxygen free. 11 Binding of Oxygen Fe II normally has ligands around it. The first 4 are occupied by binding to the four nitrogen atoms of the heme. This binding leaves 2 binding sites ring. In Mb one site is the N atom of His 93. Residue 93 is in the F helix (Called His F8 or proximal His) In Deoxy Mb, the remaining site is H 2 O. When it binds O 2 occupies this site. On the other side of the bound O 2 ,lies His 64 (E7) called the distal His Therefore O 2 is sandwiched between the heme Fe and the ring N of the distal His. There is similar binding in Hb. THE HYDROPHOBIC EN VIRON MENT IN THE PROTEIN SER VES TO PRE VENT THE OXIDATION OF Fe IIFe III 12 13 Changes in the heme group upon O2 binding to the chains of hemoglobin shown in this slide and the previous one, The heme groups of the Deoxy (T) and oxy (R) structures are shown. The two oxygen atoms of the bound O2 are shown below the heme group. Upon binding, the iron atom moves about 0.6 from above into the plane of the heme. This action pulls with it His 87, also known as F8, the 8th residue of the F helix, and the F helix of which it is a part. This in turn produces changes in the FG corner on the left, which is in contact with the other chain across the 12 interface. It is this interface which changes during the T R quaternary structure change. The above mechanism describes a plausible, but unproven sequence of events by which oxygen binding affect the quaternary structure. The dashed lines are H-bonds , presumably involved in orienting this His F8 side chain. 14 Hemoglobin can exist in two different conformations: The T-form and the R-form. The Tform is characterized by the presence of many salt linkages (or bridges) between different subunits, and these linkages are broken as hemoglobin undergoes the T --> R transition upon oxygenation. For instance, two pairs of salt bridges are present in deoxyhemoglobin, involving the C-terminal groups (Arg141) of the a-chain at the a1/a2 interface. Specifically, the negatively charged carboxyl group of Arg141 of each a-subunit makes a salt bridge with the positively charged amino group of Lys127 of the other a-subunit. And the positively charged guanidino group of Arg141 interacts electrostatically with the carboxyl group of Asp126's side chain. 15 16 A plot of the the 12 interface in the R and T states of hemoglobin as indicated , illustrating the large differences. At the top is the C helix 1 chain, at the bottom the irregular corner linking the F and G helices of the 2 chain. The conformations of both are virtually the same in both the R and T structures, but the contacts differ markedly owing to a shift of one subunit relative to the other. For example His 97 of the chain is in contact with thr 41 of the chain in T, but with thr 38, one turn back along the C helix in R. Intermediate positions would be unstable since his 97 and thr 41 would be too close together. Therefore, molecules must either be in the T or R states, irrespective of the number of bound ligands 17 F Helix Transition Recall that there is extensive contact between the alpha-beta interfaces in Hb. While the alpha1-beta1 and alpha2-beta2 interfaces are rigid, the alpha1-beta2 and alpha2-beta1 interfaces are somewhat flexible. This flexibility allows the translation of the F helix to result in a quaternary shift at the alpha1-beta2 and alpha2-beta1 interfaces. Shown above, the beta2 F helix terminus- His 97- moves down one turn of the alpha1 C helix, from in between the Pro 44 and Thr 41 residues to in between the Thr 41 and Thr 38 residues. No intermediate position would be stable- the His 97 and Thr 41 residues would bump into each other. This accounts for the absence of any stable intermediate form between the T and R states. Accompanying the F helix translation is extensive breaking and reforming of salt bridges and H- bonds, Below is a view of a portion of the alpha1-beta2 interface. It is possible to see the chain reaction of events that lead to the reorientation at the alpha1-beta2 (and alpha2-beta1) interfaces. Upon oxygen binding (not shown), the heme's doming is flattened, pulling the proximal his (here His 92) 0.6 Å towards the heme. This forces the attached F helix to undergo a 1 Å translation along the heme plane, pulling with it the attached FG helix. This movement results in the reorientation at the alpha1-beta2 interface, where the end of the F helix and the side of the FG helix are forced to break and reform many hydrogen bonds and salt bridges (The hydrogen bond between Tyr 42 and Asp 99 is one example). 18 19 The binding of oxygen rotates the globin chains, moving the ß chains together and sliding the a1ß2 interfaces apart (the R state) thus increasing the oxygen affinity of Hgb. Hemoglobin must bind O2 at high O2 tension and release it at low O2 tension. With deoxygenation the a1ß2 interface tightens lessening the affinity of Hgb for oxygen. This conformation is stabilized by proton binding and 2,3-DPG. Decreasing pH strengthens the a1ß2 interface, stabilizing the low-affinity (T) conformation and releasing O2 . This is the Bohr effect. 20 Away from the cell Fe II slowly oxidizes to become met Mb-Met proteins don’t bind O 2 . When O 2 binds, a temporary rearrangement of electrons occurs. When the O 2 is released, the Fe remains Fe II and can bind O 2 once again. To understand the function (binding and release of O 2 ),we some elementary Physical Chemistry [O 2] in fluid P Law O2 (gas). This is called Henry’s So that [O 2] is expressed as the pressure of O 2. It is easy to measure the fraction of binding sites occupied on the protein from changes in the visible absorption spectrum when the binding sites are occupied. 21 22 The Significance of the Sigmodial Oxygen Binding Curve The significance of the sigmodial O2 saturation curve of Hb can be appreciated from the graph below. While both Mb and Hb will be saturated with O2 at the partial pressure of O2 in the lungs, only hemoglobin will release significant amounts of O2 at the partial pressure of O2 present in the tissues. In fact, the O2 released by Hb can then be taken up by Mb for O2 storage in those tissues, such as muscle, that have significant amounts of Mb. 23 24 25 26 27 The different behavior of oxygen binding of myoglobin and hemoglobin can be summarized as follows. Myoglobin binds O2 under conditions where hemoglobin releases it. This is below 20 Torr in muscle tissue. At this pressure, hemoglobin releases almost all of its oxygen and myoglobin binds the freed oxygen at over 90%. The hyperbolic curve of Mb binding is typical for non-cooperative processes, while the sigmoidal curve of Hb binding is typical for cooperativity 28 Summary of binding methods for one site, one step, binding 29 Some common methods of plotting binding data, using theoretical curves constructed for the simple reaction P + A PA A) Normal hyperbolic relationship between binding and free ligand concentration. B) A logarithmic scale demonstrating the large range of ligand concentrations required for a complete binding curve. C) A Scatchard plot-The negative slope gives the value of the association constant. D) A Hill plot. Both the liganded and free protein concentrations are required for this plot. The plot is used primarily for studies of cooperative binding. 30 A. Allosterics and conformational changes Conformational changes in proteins (alterations in the typically in the tertiary /quarternary structure) are critical to their function and regulation. Changes in protein conformation facilitate movement, catalysis, release of molecules (oxygen), binding, etc. Since conformational changes are both the result of and lead to alterations in noncovalent interactions within and between protein(s), regions in proteins must be able to allow the structural changes (breaking and forming new non-covalent interactions). 31 Conformational changes which result from an interaction with a specific small molecules are often described as Allosterics. Allosteric interactions (from the Greek allos, other) = interactions or changes in conformation (shape) of a regions in a protein (or another protein) which INDUCES a change in shape in another part of the same molecule (or another molecule) Allosteric interactions typically occur when a specific small molecule, called an allosteric modulator or allosteric effector, binds to a protein (often an enzyme) and modulates its activity. The allosteric modulator typically binds reversibly at a site often separate from the functional binding site of the protein. 32 thereby 33 34 35 36 Most recent results on structure changes . How are the tertiary and quaternary structure changes related to each other ? The truth apparently lies somewhere between the MWC model and the Koshland model. The tertiary structure changes that accompany oxygen binding can be tolerated up to a certain point before the T R switch. Apparently whenever one site is occupied on each of the two dimers, the whole molecule adopts the R quaternary structure. 37 38 39 The red blood cell contains high levels of bisphosphoglycerate (BPG), which is an ALLOSTERIC EFFECTOR of Hb's affinity for O2. BPG binds strongly to the deoxy form of Hb, but only weakly to the oxy form. Thus, BPG favors the deoxy conformation (bpg). The importance of bisphosphoglycerate (BPG) as an allosteric regulator of hemoglobin's O2 affinity High altitude adaptation Adaptation to high altitude is a complex physiological process that involves many events. One event that occurs within 24 hours is an increase in the content of BPG in the erythrocyte. The effect of the increased concentration of BPG is to reduce the affininity of hemoglobin for O2, which increases the efficiency of O2 delivery to tissues The Physiological Transport of O2 and CO2 How do all these allosteric effects combine to allow Hb to carry O2 from the lungs (gills) to the tissues and to carry CO2 from the tissues to the lungs (gills)? As shown below, Hb stripped of all its allosteric effectors has to high an affinity for O2 to allow effective transport of O2 to tissues. However, the presence of both CO2 in the tissues and BPG in the red blood cell create a situation in which O2 is efficiently transported from lung to tissue. We can summarize these events as follows: 1.In the lungs the partial pressure of O2 is high, which overcomes any negative allosteric effects and causes complete oxygenation of Hb. 2.As Hb-O2 enters the tissues the presence of CO2 and a lowered pH combine to favor the deoxy conformation and release of O2. 3.The presence of BPG aids the delivery of O2 by favoring the deoxy conformation. 4.Deoxy Hb binds CO2. 5.The deoxy Hb returns to the lungs where the pH is higher, the O2 content higher and the CO2 content is lower. All these factors favor reverse of the carbamation reaction, deprotonation of His 146 and the formation of oxy Hb. 40 Genes present today are the product of evolutionary divergence. Proteins then accumulate mutations unless natural selection has acted. Overall mutation rates seem to occur at a rate of 1/100 (million years). Neutral mutation rates are a fraction of this and vary with each nucleotide and each gene. Mutations that adversely affect the gene are (presumably) selected against. The number of mutations that may be tolerated varies among genes. Cytochrome C Part of the chain of linked oxidations and reductions involved with biological electron transport. Sequences from many organisms are available. The figure below shows a difference matrix giving the number of amino acid differences in cytochrome C among a few selected eukaryotic species. 41 A composite of sequences , where every amino acid that has been found at every position is listed below. 41 species are included . The numbering system used is that of the mammalian proteins, which start at 1 and end at 104. The longest proteins included in the Table start at -8 and end at 105. Wide variation occurs at 60, 89,92. No variation is found at other positions such as 14,17, to which the heme group in this protein is attached covalently. Positions 76-80 are also invariant 42 The evolutionary process of divergence may be tracked by reconstructing a phylogenetic tree from the amino acid changes. This is a computer based calculation, since 2 million trees are possible with 10 species. Phylogenetic tree constructed from the sequences of cytochrome C. The numbers of amino acid changes along each segment are listed. 43 Mutations implied by Divergence The non-random nature of the pairs of amino acids that replace each other in a number of different proteins are shown in the matrix below. The most prevalent replacements occur in amino acids with chemically similar amino acid side chains: Gly/Ala, Ala/Ser, Ser/Thr, Ile/Val/Leu , Lys/Arg , Tyr/Phe This bias in amino acid replacements does not arise from the nature of the genetic code, even though amino acids differing by single nucleotides in their codons are most likely to replace each other. The second nucleotide in the codon is the most important in specifying the chemical nature of the amino acid; changes in the first interconvert similar amino acids, changes in the third usually produce no change in amino acid. 44 The expected distribution with random basepair changes and random use of the code is shown in the upper half of the Figure on the next page. This distribution is quite different from that actually observed The relative mutabilites of residues during evolution is shown in the Table below. 45 Rates of Divergence in Evolution The specific rates at which various proteins have evolved are shown below for fibrinopeptides A and B, cytochrome C and hemoglobin. For each protein the rate is a constant which differs from the others. How do we explain the wide range of evolutionary rates among proteins and the nearly constant rate in time for the individual proteins ? Idea: If the precise amino acid sequence was not critical for protein function, then a large fraction of the total mutations would be neutral, and the protein would evolve quite rapidly. 46 e.g. The fibrinopeptides seem to only block the aggregation of fibrinogen. At the other end of the spectrum, a protein for which very few amino acid rates are acceptable would have a very slow rate of evolution. The example is cytochrome C , which must interact with several proteins in its biological function of transferring electrons. The neutral mutation rate would be different for each position within the gene or protein. The third nucleotide base pair in many codons is not specific for the amino acid coded and is found to have evolved most rapidly. In contrast, some amino acids with vital roles can’t be changed, as we previously noted. 47 Variants in Hemoglobin Human cells carry two copies of each chromosome. If there is a normal () and mutated (*) form of the chain gene in Hb, an individual can have three possible combinations of these genes, as follows: (I) homozygous (normal) (II)* heterozygous (mixed) (III) ** homozygous variant. Individuals with will produce normal Hb chains * produces both normal and variant chains while, ** produces only variant chains. 300 Hb variants are known 48 Sickle cell disease Glu val at residue 6 of the chain causes deoxy Hb to polymerize, thereby producing the characteristic sickling. This leads to blockage of capillaries carrying blodd and a sickling crisis. Interesting issue: Why does this apparently detrimental variant persist ? -only homozygotes suffer from sickle cell anemia -heterozygotes don’t. 1/2 the Hb is normal which is sufficient to inhibit polymerization. -Sickle cell Hb confers resistance to malaria.The parasite spends part of its reproductive cycle in the erythrocyte. Possibly, the increased fragility of the cells disrupts the life cycle. - heterozygotes are evidently more fit in regions where malaria exists. 49 Other types of genetic defects: Thalassemias-one or more chains are not produced - One of the genes is deleted or has undergone a nonsense mutation - All genes may be present but transcription may be blocked Effects of thalassemias: - -thalassemia. Individuals homozygous for this defect must use fetal () chains to make 2 2 Hb. These individuals usually don’t survive to maturity. There are milder cases where transcription in only partially inhibited. The text describes another type of thalassemia. 50 51 Hemoglobin Mutants: Missense, Nonsense, and Frameshift The top line above shows the DNA code and amino acid sequence for the first 29 amino acids of the beta globin chain. The next four lines show the same information for four, different mutants. The changed base and amino acid(s) shown in red. HbS and HbC are both missense mutants at the #6 amino acid position substituting valine or lysine for glutamic acid. Hb Thalassemia is a general name for a reduction in the amount of hemoglobin produced. The first HbThal mutant shown has a Stop or Termination codon (TAG) at position #17 in place of the codon for lysine. The second HbThal mutant results from the deletion of two adjacent adenines at position #8 causing a shift in the reading frame to produce a series of missense amino acids terminating at a now inphase, early termination codon. 52