* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Relationship between the structure and function of proteins
Peptide synthesis wikipedia , lookup
List of types of proteins wikipedia , lookup
Radical (chemistry) wikipedia , lookup
Gaseous signaling molecules wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Protein adsorption wikipedia , lookup
Bottromycin wikipedia , lookup
Genetic code wikipedia , lookup
Circular dichroism wikipedia , lookup
Ligand binding assay wikipedia , lookup
Drug design wikipedia , lookup
Expanded genetic code wikipedia , lookup
Amino acid synthesis wikipedia , lookup
Proteolysis wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Point mutation wikipedia , lookup
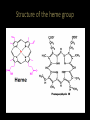
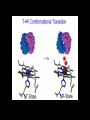
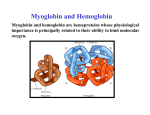
Relationship between the structure and function of proteins Myoglobin Myoglobin • • • • • • • • It is located in the muscle tissue and is responsible for its brown color. Its function is to store and transport oxygen in the skeletal muscles. It is a relatively small protein made up of a single polypeptide chain that contains 153 amino acid residues . It contains a heme group (which is a prosthetic group consisting of a protoporphyrin organic ring and a central iron atom). It is the heme group which is responsible for the oxygen binding capacity of Myoglobin. Myoglobin is very similar to Hemoglobin in both its function and structure ( since both are capable of oxygenation and deoxygenation). Myoglobin is an extremely compact molecule(in its interior there is room for only 4 H2O molecules) The backbone of the polypeptide chain is made up of 8 segments of α-helical structures , the largest segment has 23 a.a while the shortest 7 a.a residues . • Thus 70% of the main polypeptide chain in myoglobin is involved in α-helical structures. • The rest of the polypeptide chain is loops or bends between the α-helical segments. • The non-polar amino acids are arranged in the interior (e.g Leu, Val, Phe)thus producing a hydrophobic interior. • The polar or charged amino acids( e.g Glu, Asp , ) are located on the outer surface , except for 2 His residues which are in the interior since they play a critical role in binding iron. Binding of the heme group • The heme group of myoglobin sits in a cleft in the interior of the molecule lined with nonpolar a.a (except for the 2 His). • There are 2 His in the interior of the myoglobin molecule , the proximal His which binds directly to the iron atom , the second His does not interact directly with the heme group , but helps stabilize the binding of the O2 to the ferrous atom. Binding of the heme group Hemoglobin Hemoglobin • Hemoglobin is an oligomeric protein which is composed of 4 polypeptide chains . • Each poypeptide chain has a heme prosthetic group attached to it in which the iron atom is in the fe2+ state . • The protein part of hemoglobin is called globin which consists of 2 α chains (141 amino acid residue) and 2β chains (146 amino acid residues). • It is roughly spherical with a 5.5 nm diameter. • The single polypeptide chain (subunit) resembles in its structure the structure of myoglobin , thus the α and β chains contain several segments of αhelix separated by bends. • Each heme is partially burried in a hydrophobic pocket lined with non-polar amino acids. • The heme group is bound to its poypeptide chain through a coordination bond of the iron atom to the R- group of the proximal His residue. The Heme • It consists of a complex organic ring structure (protoporphyrin) which is a tetrapyrrol ring linked by four methene bridges ( =CH groups) . • It contains methyl , vinyl ,propionic acid groups attached to the pyrrol rings. • The non-polar vinyl group is burried in the hydrophobic interior while the hydrophilic groups of the propionic acid projects out of the pocket to the outside. • The iron atom has 6 coordination bonds . • Four in the plane of the portophyrin ring and attached to it by binding to the 4 central nitrogen atoms , the two remaining coordination bonds are perpendicular to the heme plane where one will bind to nitrogen atom of the proximal His residue and the other will bind the O2 molecule. Structure of the heme group Hemoglobin Hemoglobin undergoes conformational changes on binding oxygen; The Hemoglobin molecule can presume two major conformations, the R-state (relaxed state) , and the Tstate (tensed state). Although Oxygen can bind to both conformations but it shows a significantly higher affinity towards the Rstate. The T-state is stabilized by a number of ionic bonds between the (α1β1 ) and (α2β2 ) dimers. The binding of oxygen to a hemoglobin subunit in the Tstate triggers a conformational change to the favored R-state , through the breaking of some of the ionic bonds with the formation of some new ones. • By changing its conformation, hemoglobin is able to bind and release oxygen molecules. • The T → R conformational transition of hemoglobin, involving its quaternary structure is strictly related to the cooperative behaviour of this protein in its reaction with oxygen. • The configuration of low affinity, deoxygenated hemoglobin (Hb) is known as the tense (T) state. Conversely, the configuration of the fully oxygenated high affinity form of hemoglobin (HbO2) is known as the relaxed (R) state. Spectral properties of HB • When there is no oxygen bound to the HEME, Hb absorbs at 430 nm, this is referred to as DeoxyHemoglobin (DeoxyHb). • When DeoxyHb bind to Oxygen, Hb absorbs at 415 nm, this is referred to as OxyHemoglobin (OxyHb). • The fractional O2 saturation Y can be calculated from this data Binding of oxygen to myoglobin and hemoglobin Hemoglobin The Bohr Effect; • The effect of pH on the O2 - Hb equilibrium is called the Bohr effect ; • HHb + O2 HbO2 + H+ . • When Hb is oxygenated it ionizes to free one H+ for each O2 bound as seen in the equation. • Since the reaction is a reversible reaction , increasing the [ H+ ] will cause the equilibrium to shift to the left releasing the O2 . • The relationship between pH and the Hb %Oxygen saturation is directly related . Hemoglobin • This pH effect and the sigmoidal property of Hb allows it to carry O2 Efficiently . • In the lungs where the partial pressure of O2 is high approximately 100 mm Hg , and the pH is also high 7.4 ,the environment favors the binding of O2 . • Whereas at the tissues where the pO2 is lower and the pH is lower deoxygenation of Hb is favored.