* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Complex IV
Mitochondrion wikipedia , lookup
Multi-state modeling of biomolecules wikipedia , lookup
Photosynthesis wikipedia , lookup
Metalloprotein wikipedia , lookup
Nicotinamide adenine dinucleotide wikipedia , lookup
Biochemistry wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Microbial metabolism wikipedia , lookup
Citric acid cycle wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Light-dependent reactions wikipedia , lookup
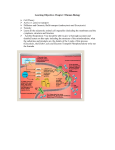
Electron transport chain wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Bioenergetics and oxidative Phosphorylation Objectives -To understand the general concepts of Bioenergetics; Enthalpy, entropy and free energy change and standard free energy and the mathematical relation between them -To understand the concepts of high energy compounds and know the most important examples, like ATP - To know the general concept of oxidative phosphorylation and the general mechanism of ATP production. Bioenergetics -Enthalpy, entropy and free energy - Free energy change and standard free energy change and relationship between them and the equilibrium constant - ATP is the universal energy carrier in the biological systems -Structural basis of the high phosphate group transfer potential -Phosphorylated compound with high phosphate group transfer potential, PEP, phosphocreatine -ATP has an intermediate group-transfer potential Oxidative Phosphorylation -Electron carriers, NADH and FADH2 -Mitochondria are the respiratory organelles in the cell -NADH dehydrogenase has two prosthetic groups FMN and iron-sulfur cluster -QH2 is the entry for electrons from FADH2 -Cytochrome Reductase -Cytochrome oxidase catalyses the transfer of electrons from CytC to O2 -Chemiosmaotic hypothesis in the phosphorylation of ADP Bioenergetics & Thermodynamics Bioenergetics: is the quantitative study of energy transduction in the living cells and nature and the chemical process underlying these transductions . •Bioenergetics concerns only with the initial and final energy states of reaction components, NOT the mechanism of the reaction, Not, the time needed for the reaction to occur. It allows to predict the spontaneousity of the reaction, wither a reaction will take place or not Factors that determine the direction of a reaction The direction and extent to which a chemical reaction proceeds is determined by two factors - Enthalpy - Entropy - Temp - Enthalpy: DH, a measure of the change in heat reaction content of the reactant and product. ∆H= H2(product)- H1(reactant) ∆H +ve Endothermic ∆H -ve Exothermic -systems tend to go forward to a lowest-energy state e.g: fall goes downhill, oxidation of fatty acids produce a lot of energy, these are spontaneous reactions and ∆H is negative, but the melting of Ice is a spontaneous reaction even it is endothermic reaction and ∆H is positive ∆H alone is not sufficient to predict the direction of a reaction -Entropy (∆S): a measure of randomness or disorder of the reactants and products. -systems have a natural tendency to randomize and the degree of randomness of a system is defined as S which is the entropy. ∆S= S2(product)-S1(reactant) ∆S +ve Increased entropy ∆S -ve Decreased entropy Entropy (∆S): a measure of randomness or disorder of the reactants and products. -Systems tend to increase the entropy (∆S +ve), e.g. Homogenization of sucrose solution with water. Entropy of ordered state is lower than that of the disordered state of the same system. Neither the entropy nor the enthalpy alone can predict the direction of the reaction. •Free Energy: Gibbs free energy that correlates the entropy and the enthalpy mathematically which allow to predict in which direction a reaction proceeds spontaneously. •Free Energy change, ∆G, Predicts the change in the free energy and thus direction of reaction at any specified concentration of products and reactants ∆G = ∆ H - T ∆ S If ∆G is –ve, the reaction proceeds spontaneously. •Standard Free energy change: ∆Gº: Free energy change under standard conditions; that when reactant and product concentration are kept at 1M conc. •The sign of the ∆G predicts the direction of the reaction. ∆G is –ve exergonic reaction. ∆G is +ve endergonic reaction. ∆G is zero equilibrium. DG of the forward and back reactions A B ∆G= -500 cal/mol, spontaneous in this direction The back reaction B A ∆G= 500 cal/mol non-spontaneous at this direction ∆G depends on the concentration of both reactants and products. For a reaction A ↔ B A: the reactants B: the product [B] ΔG ΔG RTln [A] o The sign of DG and DGº can be different ∆Gº gives prediction of the direction of the reaction only at the standard conditions: At standard conditions the [A]=[B]=1 ∆G = ∆Gº + RTln1 ∆G = ∆Gº at standard conditions Relation between equilibrium constant (Keq) and ∆Gº A ↔ K B eq at equilibrium [B] eq [A]eq ΔG ΔG RTln o [B] eq [A]eq at equilibrium DG=0 0 ΔG o RTln ΔG RTlnk [B] eq [A] eq o eq If Keq=1 DGº=0 If Keq>1 DGº < 0 (-ve) If Keq<1 DGº > 0 (+ve) Free Energy change profile ∆G is –ve exergonic reaction spontaneous from A to B ∆G is +ve endergonic reaction non-spontaneous from B to A The reaction of Glucose 6-PO4 into Fructose 6-PO4 under different conditions Glucose 6-PO4 ↔ Fructose 6-PO4 Glucose 6-PO4 Fructose 6-PO4 Fructose 6-PO4 Glucose 6-PO4 Glucose 6-PO4 ↔ Fructose 6-PO4 ∆Gº of two consecutive reactions are additives and also DG of pathways are additives Reactions or processes with a large +ve ∆Gº as moving against electrochemical gradient are made possible by coupling the endergonic process with a large –ve process as hydrolysis of ATP Favorable and unfavorable reactions are coupled through common intermediates A+BC+D DF A + B + DC+ D+ F A+BC+F ∆Gº1 (non-spontaneous) ∆Gº2 (spontaneous) ∆Gº3= ∆Gº1 + ∆Gº2 (spontaneous) D is a common intermediate and can serve as energy carriers for this reaction •ATP is the universal energy carrier in biological systems ATP: nucleotide consists of adenine, ribose and triphosphate unit, the active form of ATP is complex with Mg+2 or Mn+2 ATP is energy rich molecule because of its triphosphate unit that contain 2 phosphanhydrid bonds, large free energy is released when ATP is hydrolyzed to ADP + Pi or to AMP and PPi ↔ ADP + Pi + H+ ATP + H2O ↔ AMP + PPi + H+ ATP + H2O ∆Gº= -7.3kcal/mol ∆Gº= -7.3kcal/mol The free energy liberated in the hydrolysis of ATP is used to drive reactions that requires an input of free energy ATP is form from ADP and Pi when fuel molecules are oxidized ATP-ADP cycle: the energy exchange in biological system Motion Biosynthesis Active transport Signal amplification ATP is continuously formed and consumed ATP ADP Photosynthesis Oxidation of Fuel molecules -Some biosynthesis reactions are driven by nucleotide analogous to ATP and these are: Gaunosine triphosphate: GTP Cytidine triphosphate: CTP Uridine triphosphate: UTP ATP + GDP ADP + GTP Structural basis of the high P group transfer potential of ATP ATP + H2O↔ ADP + Pi + H+ Glycerol 3-phosphate + H2O ↔ Glycerol + Pi ATP has a stronger tendency to transfer its terminal phosphoryl group to water than dose the glycerol 3-phosphate ATP has high phosphate group transfer potentials Why? 1- Electrostatic repulsion 2- Resonance stabilization ∆Gº = -7.3 kcal/mol ∆Gº= -2.2 kcal/mol Other compounds have high phosphate group transfer potential -Phosphoenol pyruvate (PEP), phosphocreatine have a higher group transfer potential than dose ATP. PEP can donate P to ADP to produce ATP PEP ↔ pyruvate + Pi ∆Gº = -62 kj/mol ADP + Pi ↔ ATP ∆Gº = +13 kj/mol PEP + ADP ↔ Pyrovate +ATP ∆Gº = -49 kj/mol ?????? It is significant that ATP has a group-transfer potential that is intermediate among the biological important phosphorylated molecules. This intermediate position enable ATP to function efficiently as a carrier of phosphoryl groups. NO enzyme in cells that transfer P from high-P donor to low energy acceptor should first transfer first to ATP to form ADP ATP is continuously formed and consumed ATP is intermediate donor of free energy in biological systems rather than as longterm storage form of energy ATP molecule is consumed after 1 min of its formation and the turnover of ATP is high, human consumes about 40 kgs of ATPs in 24 hr ATP hydrolysis is coupled to reaction to shift the reaction toward product AB (non-spontaneous) ∆Gº = +4 kcal/mol ATP + H2O ADP + Pi ∆Gº = -7.4 kcal/mol A + ATP + H2O B + ADP + Pi + H+ DGº = -3.4 kcal/mol Spontaneous * NAD+ is the oxidized form of nicotinamide adenine dinucleotide, NADH is the reduced form NADH: generation of ATP NADPH: reductive biosynthesis * NAD+ is the major eacceptor in oxidation of fuel molecules. Oxidizing agent reducing agent NAD+ + 2e- + H+ NADH Oxidized form reduced form NAD+ is strong oxidizing agent that can oxidize secondary alcohol into keton * electron donor= reducing agent (reductant) electron acceptor= oxidizing agent (oxidant) * Flavin adenine dinucleotide (electron carrier molecule) FAD: Oxidized Form FADH2: Reduced Form FAD + 2e- + 2H+ Oxidizing agent Oxidant FADH2 reducing agent reductant FAD is strong oxidizing agent it can oxidize the alkain into alkene Oxidative Phosphorylation Oxidative phosphorylation: is the process in which ATP is formed as a result of transfer of electrons from NADH or FADH2 to O2 by a series of electron carriers NADH, FADH2 formed glycolysis, Fatty acid oxidation and citric acid cycle. They have a pair of electrons with high transfer potential when these electrons are transferred to O2, a large free energy is librated The flow of electrons from NADH or FADH2 to O2 through protein complexes in the inner membrane of the mitochondria leads to pumping of protons out the mitochondrial matrix, this makes pH and Transmembrane electrical gradient ATP is synthesized when proton flow to the mitochondrial matrix O2 H2O ATP ADP + Pi -- - - ++++ H+ H+ •The Respiratory chain consists of three proton pumps, linked by two mobile electron carriers. • electrons are transferred from NADH to O2 through a chain of three large protein complexes Complex I:NADH dehydrogenase, NADH-Q reductase. Complex III: Cytochrome reductase. Complex IV: Cytochrome oxidase. The above protein complexes are pump protons Ubiquinone (Q): carries electrons from NADH dehydrogenase (I) to cytochrome reductase (III) Cytochrome C: carries electrons from cyt-reductase (III) to cytochrome oxidase (IV). Complex II: succinate dehydrogenase, (succinate- Q reductase), doesn't pump protons, production of FADH2 from succinate NADH Complex I NADH dehydrogenase Complex II Q Ubiquinone (Q), Cytochrome C are mobile e-carriers FADH2 Cytochrome reductase. FAD Complex III Cytochrome C Cytochrome oxidase. O2 Complex IV NADH dehydrogenase. Complex I Complex II succinate dehydrogenase (Succinate-Q oxireductase) Cytochrome reductase. Complex III Cytochrome Oxidase. Complex IV Oxidative Phosphorylation NADH dehydrogenase.(Complex I) The electrons of NADH enter the chain at the NADH dehydrogenase, the initial step is the binding of NADH and then the transfer the two electrons to the flavin mono nucleotide (FMN) prosthetic group of this protein to give the reduced form FMNH2 NADH + H+ + FMN FMNH2 +NAD+ Electrons transfer from the Fe-S cluster of complex I are shuttled to Coenzyme Q NADH NAD+ FMN FMNH2 The flow of two electrons from NADH to QH2 leads to pumping of four H+ from the matrix to intermembrane space reduced Fe-S oxidized Fe-S Q QH2 QH2 shuttle electrons from complex I to cytochrome reductase (complex III) It is hydrophobic quinone diffuse rapidly within the inner membrane of mitochondria Oxidized form of Q Intermediate Reduced form Electrons flow from Ubiqinol to cyto. C through Cytochrome reductase Cytochrome is an electron-transferring proteins that contain a heme prosthetic group. Their iron atoms alternate between a educed ferrous(+2) state and an oxidized ferric(+3) state during electron transport. Cyt. Reductase catalyzes the transfer of 2 e- from QH2 to Cyt. C (water soluble protein) and this is coupled to pumping of 4 H+ to the inter-membrane space * Cyt. Reductase has two types of cytochromes; b and C1 Cyt. C1 and Cyt. C have ironprotoporphyrine1X the same as heme of myoglobin, hemoglobin and these hemes are covalently linked to protein Cytochrome reductase. Complex III QH2 transfer one of its electrons to Fe-S cluster in the reductase. Then this electron is shuttled to Cyt. C1 then to Cyt. C which carries it away from the complex Complex IV: Cytochrome Oxidase. Cytochrome Oxidase catalyses the transfer of electrons from Cyt C to O2 In this reaction 2Cyt C(+2) + 2H+ +1/2 O2 2Cyt C(+3) +H2O This process accomplished by pumping 2 protons from matrix to intermembrane space Cytochrome Oxidase contains two heme A groups called heme a and heme a3 , they are different because they differ in their location in the location. Cyt. Oxidase contains also two copper ions called CuA and CuB as prosthetic group O2 is reduced into water Complex II: succinate dehydrogenase (Succinate-Q oxireductase) QH2 is the entry for electrons from FADH2 of Flavoproteins FADH2 is formed in citric acid cycle by the oxidation of the succinate to fumarate by succinate dehydrogenase (complex II) which is integral protein in the mitochondrial inner membrane, FADH2 doesn't leave the complex, but its electrons are transferred to Fe-S cluster then to Q for the entry to the electron transport chain, the same thing for the FADH2 moieties of glycerol dehydrogenase, and Fatty acyl Co dehydrogenase transfer their high potential electrons to Q to from QH2, these enzymes are not proton pumps * Oxidation and Phosphorylation are coupled by a proton-motive force NADH + ½ O2 + H+ H2O + NAD+ ∆G0= -52.6 kcal/mol ADP + Pi + H+ ATP + H2O ∆G0= +7.3 kcal/mol * ATP synthesis is mediated by mitochondrial ATPase (ATP synthase in the inner membrane of mitochondria) * Oxidation of NADH is coupled to Phosphorylation of ADP into ATP The chemiosmotic hypothesis The transfer of electrons through the respiratory chain leads to pumping of protons from the matrix to the other side of the inner mitochondrial membrane. The H+ concentration becomes higher on the systolic side and the electrical potential is generated and this proton motive force drives the synthesis of ATP by the ATPsynthase complex * Oxidation of 1 NADH 3ATP 1 FADH2 2ATP Oxidative Phosphorylation * Electrons transfer in the respiratory chain can be blocked by specific inhibitors * Oligomycin: drug bind to ATP synthase that prevents the rentry of H+ prevents ATP synthesis prevent electron transport so electron transport and phosphorylation are coupled The End iron-sulfur cluster (Fe-S) in iron-sulfur proteins (non-hem proteins) play a critical role in a wide range of reduction reactions in biological systems, three types of Fe-S cluster: The simplest one is consisting of one iron atom coordinated to 4 sulfhydryl group of four cysteine molecules A second type [2Fe-2S] Third type [4Fe-4S] NADH Dehydrogenase contain the [2Fe-2S] and [4Fe-4S] Iron atoms in these clusters cycle between Fe (reduced ) and Fe (oxidized )