* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download peptides - WordPress.com
Survey
Document related concepts
Basal metabolic rate wikipedia , lookup
Metalloprotein wikipedia , lookup
Photosynthesis wikipedia , lookup
Electron transport chain wikipedia , lookup
Microbial metabolism wikipedia , lookup
Biochemistry wikipedia , lookup
Light-dependent reactions wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Citric acid cycle wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Transcript
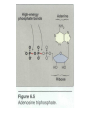
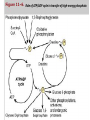
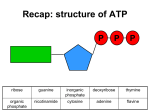
in our bodies , the conversion of metabolite A to metabolite B with release of free energy is coupled to another reaction in which free energy is required to convert metabolite C to D. Many coupled reactions use ATP to generate a common intermediate. These reactions may involve ATP cleavage—that is, the transfer of a phosphate group from ATP to another molecule. Other reactions lead to ATP synthesis by transfer of phosphate from an energy-rich intermediate to ADP, forming ATP. In order to maintain living processes, all organisms must obtain supplies of free energy from their environment. Generally, human beings obtain energy by coupling their metabolism to the breakdown of complex organic molecules in their environments. ATP plays a central role in the transference of free energy from the exergonic to endorgonic reactions. Reactions or processes that have a large positive ∆G, such as moving ions against a concentration gradient across a cell membrane, are made possible by coupling the endergonic movement of ions with a second spontaneous process with a large negative ∆G, such as the hydrolysis of adenosine triphosphate (ATP). ATP consists of a molecule of adenosine (adenine + ribose) to which three phosphate groups are attached. Three phosphoryl groups are sequentially linked to the fifth position of a ribose moiety via the phosphoester bond, followed by two phosphoanhydride bonds. Two terminal phosphorly groups, i.e the ɣ and β phosphoryl groups, are involved in phosphoric acid anhydride bonding and are designated as energy-rich bonds or high-energy bonds, which are symbolised (῀ ) If one phosphate is removed, adenosine diphosphate (ADP) is produced; if two phosphates are removed, adenosine monophosphate (AMP) results. The standard free energy of hydrolysis of ATP, ∆G°, is approximately -7.3 K cal/mol for each of the two terminal phosphate groups. Because of this large, negative ∆G°, ATP is called a high-energy phosphate compound. the second reaction (ADP→AMP+Pi) is of less biological importance than the ATP to ADP hydrolysis. ATP act as energy transporter (energy currency), it can provide energy through hydrolysis and release of phosphate groups to force endergonic reactions to take place, to provide mechanical energy (muscle movement) and heat energy ( to maintain body temperature). It is also possible to go directly from ATP to AMP by cleaving a pyrophosphate group (but the produced energy for such reaction is only -10.9k cal/ molecule). ATP → AMP + PPi Note 1: not only ATP act as energy transporter but other nucleotides also can play the same function like GTP and UTP and produce the same energy as ATP. Note2: in our bodies there are different high energy molecules that when catabolized can yields energy to be transported to the site of utilization by ATPs e.g1:phosphoenolpyruvate can yields(-14.8 Kcal/molec.), 1,3 bisphosphoglycerate (-11.8 Kcal/molec.) And acetyl phosphate (-10.3 Kcal/molec.) which are important for the conservation of energy. e.g2: phosphocreatine (-10.3 Kcal/molec.) is stored in muscles and can rapidly converted to ATP to supply muscle contractions. Production of phosphocreatine occurs when ATP concentration is high and the reverse occur when ATP concentration is low. ATP ADP+phosphocreatine +creatine Table 11–1. Standard Free Energy of Hydrolysis of Some Organophosphates of Biochemical Importance High-Energy Phosphates Are Designated by The symbol indicates that the group attached to the bond, on transfer to an appropriate acceptor, results in transfer of the larger quantity of free energy. Thus, ATP contains two high-energy phosphate groups and ADP contains one, whereas the phosphate in AMP (adenosine monophosphate) is of the lowenergy type, since it is a normal ester link (Figure 11–5). Structure of ATP, ADP, and AMP showing the position and the number of high-energy phosphates ( ). HIGH-ENERGY PHOSPHATES ACT AS THE "ENERGY CURRENCY" OF THE CELL ATP is able to act as a donor of high-energy phosphate to form those compounds below it in Table 11–1. Likewise, with the necessary enzymes, ADP can accept high-energy phosphate to form ATP from those compounds above ATP in the table. In effect, an ATP/ADP cycle connects those processes that generate P to those processes that utilize (Figure 11–6), continuously consuming and regenerating ATP. This occurs at a very rapid rate, since the total ATP/ADP pool is extremely small and sufficient to maintain an active tissue for only a few seconds. Role of ATP/ADP cycle in transfer of high-energy phosphate. There are three major sources of taking part in energy conservation or energy capture: 1.Oxidative phosphorylation: The greatest quantitative source of in aerobic organisms. Free energy comes from respiratory chain oxidation using molecular O2 within mitochondria. 2.Glycolysis: A net formation of two results from the formation of lactate from one molecule of glucose, generated in two reactions catalyzed by phosphoglycerate kinase and pyruvate kinase, respectively . 3.The citric acid cycle: One is generated directly in the cycle at the succinate thiokinase step. When ATP acts as a phosphate donor to form those compounds of lower free energy of hydrolysis (Table11–1), the phosphate group is invariably converted to one of low energy, eg, ATP Allows the Coupling of Thermodynamically Unfavorable Reactions to Favorable Ones • The phosphorylation of glucose to glucose 6phosphate, the first reaction of glycolysis , is highly ender-gonic and cannot proceed under physiologic conditions. To take place, the reaction must be coupled with another—more exergonic—reaction such as the hydrolysis of the terminal phosphate of ATP. When (1) and (2) are coupled in a reaction catalyzed by hexokinase, phosphorylation of glucose readily. proceeds in a highly exergonic reaction that under physiologic conditions is irreversible. Many "activation“ reactions follow this pattern. Adenylyl Kinase (Myokinase) Interconverts Adenine Nucleotides This enzyme is present in most cells. It catalyzes the following reaction: This allows: 1. High-energy phosphate in ADP to be used in the synthesis of ATP. 2. AMP, formed as a consequence of several activating reactions involving ATP, to be recovered by rephosphorylation to ADP. 3. AMP to increase in concentration when ATP becomes depleted and act as a metabolic (allosteric) signal to increase the rate of catabolic reactions, which in turn lead to the generation of more ATP . When ATP Forms AMP, Inorganic Pyrophosphate (PPi) Is Produced ATP can also be hydrolyzed directly to AMP, with the release of PPi (Table 11–1). This occurs, for example, in the activation of long-chain fatty acids: This reaction is accompanied by loss of free energy as heat, which ensures that the activation reaction will go to the right; and is further aided by the hydrolytic splitting of PPi, catalyzed by inorganic pyrophosphatase, a reaction that itself has a large ∆G° of –19.2 kJ/mol. Note that activations via the pyrophosphate pathway result in the loss of two rather than one, as occurs when ADP and Pi are formed. A combination of the above reactions makes it possible for phosphate to be recycled and the adenine nucleotides to inter-change (Figure 11– 8). Other Nucleoside Triphosphates Participate in the Transfer of High-Energy Phosphate By means of the enzyme nucleoside diphosphate kinase, UTP, GTP, and CTP can be synthesized from their diphosphates, eg, All of these triphosphates take part in phosphorylations in the cell. Similarly, specific nucleoside monophosphate kinases catalyze the formation of nucleoside diphosphates from the corresponding monophosphates. Thus, adenylyl kinase is a specialized monophosphate kinase. Chemically, oxidation is defined as the removal of electron(s) and reduction is defined as the gain of electron(s). biological oxidation ( which mean the oxidation that occurs in biological systems ) is very important to supply the body with energy. Oxidation in biological systems can occur by : 1. removal of electrons . 2. removal of hydrogen . 3. addition of oxygen (less common). Electrons are not stable in free state, so their removal from a substance (oxidation) must be accompanied by their acceptance by another substance (reduction) hence the reaction is called oxidation reduction reaction or redox reaction and the involved enzymes in the catalyzing of the reaction are called oxidoreductases. Redox potential: it is the affinity of a substance to accept electrons i.e it is the potential for a substance to become reduced. Hydrogen has the lowest redox potential (-0.42 volt ) while oxygen has the highest redox potential (+0.82 volt). The redox potential of all other substances lie between that of hydrogen and oxygen. Electrons are transferred from substances with low redox potential to substances with higher redox potential . this transfer of electrons is an energy yielding process and the amount of energy librated depends on the redox potential difference between the electron donor and acceptor. Oxido-reductases: These are group of enzymes that catalyze oxidation reduction reactions which include 4 classes. 1. oxidases. 2. dehydrogenases 3. hydroxy peroxidases 4. oxygenases. 1-Oxidases: they catalyze the removal of hydrogen from a substrate using oxygen as a hydrogen acceptor, they form water (H20) or hydrogen peroxidase (H2O2) as a reaction product. e.g : cytochrome oxidase is a hemo protein widely distributed in many tissues, having a typical heme prosthatic group (which also present in other cytochromes, myoglobin and hemoglobin). Cytochrome oxidase is an important member of the respiratory chain which found in the mitochondria , it is also called cytochrome a3 or aa3. Note: some of the oxidases cannot catalyze the direct redox reactions but only with the help of transporters (FMN+ and FAD+ co enzymes ) which act to transfer the reducing equivalents from the substrate to the acceptor , such enzymes are called the flavoprotin oxidases. In these enzymes FMN (flavin mono nucleotide) or FAD (flavin adenine di nucleotide) presented as prosthetic group that tightly bound to their enzyme. Different flavoproteins are presented in our bodies like L-amino acid oxidase which is FMN linked enzyme found in the kidney which is important for the oxidative metabolism of Lamino acids. Note : some of these flavoproteins contain one of the metals as essential co-factor and such enzymes is called metalloflavoproteins like : xanthine oxidase ( contain molybdenum) and plays an important role in the conversion of purine base to uric acid.