* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Dissection of molecular interactions of replication protein A in
Survey
Document related concepts
Transcript
University of Iowa
Iowa Research Online
Theses and Dissertations
Fall 2015
Dissection of molecular interactions of replication
protein A in replication and repair
Ran Chen
University of Iowa
Copyright © 2015 Ran Chen
This dissertation is available at Iowa Research Online: http://ir.uiowa.edu/etd/2192
Recommended Citation
Chen, Ran. "Dissection of molecular interactions of replication protein A in replication and repair." PhD (Doctor of Philosophy)
thesis, University of Iowa, 2015.
http://ir.uiowa.edu/etd/2192.
Follow this and additional works at: http://ir.uiowa.edu/etd
Part of the Biochemistry Commons
DISSECTION OF MOLECULAR INTERACTIONS OF REPLICATION PROTEIN A
IN REPLICATION AND REPAIR
by
Ran Chen
A thesis submitted in partial fulfillment
of the requirements for the Doctor of
Philosophy degree in Biochemistry
in the Graduate College of
The University of Iowa
December 2015
Thesis Supervisor: Professor Marc S. Wold
Copyright by
RAN CHEN
2015
All Rights Reserved
Graduate College
The University of Iowa
Iowa City, Iowa
CERTIFICATE OF APPROVAL
_______________________
PH.D. THESIS
_______________
This is to certify that the Ph.D. thesis of
Ran Chen
has been approved by the Examining Committee
for the thesis requirement for the Doctor of Philosophy
degree in Biochemistry at the December 2015 graduation.
Thesis Committee: ___________________________________
Marc S. Wold, Thesis Supervisor
___________________________________
Todd Washington
___________________________________
Daniel L. Weeks
___________________________________
Ernesto J. Fuentes
___________________________________
David H. Price
___________________________________
Aloysius Klingelhutz
To my parents who always support me and give me strength
To my mentor who always patiently guided me and taught me not to be scared of
unexpected results from experiments
To my friends who always help me and encourage me, like family members
ii
Give a man a fish and you feed him for a day; teach a man to fish and he will eat forever.
Chinese Proverb
iii
ACKNOWLEDGMENTS
Thank you to Marc Wold for valuable discussion and insights on the work
presented here.
iv
ABSTRACT
Replication protein A (RPA) is the major eukaryotic single-strand DNA (ssDNA)
binding protein. RPA is composed of three subunits, RPA1, RPA2 and RPA3. RPA is
essential for replication, repair, recombination, and checkpoint activation, and is required
for maintaining genome integrity. In the cell, RPA binds to ssDNA intermediates and
ensures that the appropriate pathway correctly processes them. The ssDNA-binding
activity of RPA is primarily mediated by two high-affinity domains in the RPA1 subunit.
DNA binds to these domains by interacting with polar and aromatic residues in a DNAbinding cleft in each domain. The aromatic residues are highly conserved and when
mutated cause a separation-of-function phenotype.
Mutation of the conserved aromatic residues in the high-affinity binding domains
of RPA only modestly affected the affinity of RPA but these aromatic residue mutants
were unable to support DNA repair while functioning in DNA replication. To understand
the molecular basis of this phenotype, I have characterized the interactions of the
aromatic mutants with different length ssDNAs and partial duplex DNA structures like
those found in DNA repair. I also probed the conformations and dynamics of RPA-DNA
complexes. My studies identified that there are at least two kinetic states when RPA
binds to ssDNA that differ in their rate of dissociation from the DNA. I also showed that
the aromatic residues are required for the stable binding to short ssDNA and contribute to
the formation of the more long-lived state. My studies also showed that the more stable
state is important for RPA in melting secondary DNA structure. We conclude that
melting activity and/or stable binding by RPA is required for DNA repair but dispensable
for DNA replication. These studies enhance our understating of molecular interactions
between RPA and DNA that contribute to different cellular functions.
The kinetic states in RPA could reflect changes in domain interactions or changes
in conformation of the RPA-DNA complex. To try to understand the molecular basis of
the different kinetic states, I used single molecule FRET analysis to characterize the
v
spatial location of RPA domains and conformational dynamics in RPA-DNA complex.
My studies showed RPA binds different locations along ssDNA and that generally RPA
does not undergo global changes in conformation when bound to ssDNA. However, with
a subset of label locations, some RPA-DNA complexes showed rare changes in
conformation. These observations were most consistent with partial microscopic
dissociation (domains of RPA partially dissociate from DNA, but has not yet equilibrated
with the surrounding solution) of domains of RPA near the 3’ end of the complex and
interactions of the flexible N-terminal, regulatory domain of RPA with the free DNA. My
data suggests that the microscopic dissociation can occur without affecting the global
structure of the RPA-DNA complex.
These studies illustrate that different DNA metabolic pathways require different
types of RPA-DNA complexes and that high affinity binding is not sufficient for all RPA
functions. Specifically, my studies showed that DNA repair pathways require different
ssDNA interactions. This suggests that modulation of the binding of individual domains
and/or inter-domain interactions regulates the properties of the RPA-DNA complex and,
in turn, that this could direct ssDNA intermediates into different pathways for processing.
Together, my studies highlight the importance of dynamics in RPA binding to properly
maintain the integrity of the genome.
vi
PUBLIC ABSTRACT
Replication protein A (RPA) is the major eukaryotic single-strand DNA (ssDNA)
binding protein. RPA is composed of three subunits, RPA1, RPA2 and RPA3. PRA is
essential for replication, repair, recombination, and checkpoint activation. In the cell,
RPA binds to ssDNA during these cellular processes. RPA protects and processes ssDNA
to maintain genome integrity. Two high affinity domains in the RPA1 subunit primarily
mediate the binding activity of RPA. Major interactions between RPA and DNA are
mediated by the polar and aromatic residues in a binding cleft of each domain.
Previous studies showed that mutation of the conserved aromatic residues in the
high-affinity binding domains of RPA only modestly affected the affinity of RPA to
DNA and that these aromatic residue mutants were unable to support DNA repair while
functioning in DNA replication. To understand the molecular basis of this phenotype, I
have characterized the interactions of the aromatic mutants with different length ssDNAs
and partial duplex DNA structures like those found in DNA repair. I showed that the
interactions mediated by aromatic residues are required for the stable binding to short
ssDNA and contribute to the formation of a more long-lived complex. My studies also
show that the stable complex is important for RPA melting secondary DNA structures.
We concluded that melting activity and/or stable binding by RPA is required for DNA
repair but dispensable for DNA replication. These studies enhance our understating of
molecular interactions between RPA and DNA that contribute to different cellular
functions.
vii
TABLE OF CONTENTS
LIST OF TABLES ............................................................................................................. xi LIST OF FIGURES .......................................................................................................... xii LIST OF ABBREVIATIONS .......................................................................................... xiv CHAPTER 1 INTRODUCTION ........................................................................................1 Eukaryotic single-strand DNA-binding proteins ..............................................2 RPA structure....................................................................................................3 RPA4 and Alternative RPA complex ...............................................................4 DNA binding modes of RPA ............................................................................6 Cellular functions of RPA ................................................................................7 RPA in DNA replication ...........................................................................7 RPA in DNA repair ...................................................................................7 RPA and DNA damage response ..............................................................9 RPA interacts with ssDNA intermediates in different cellular pathways.......10 Cellular RPA levels, genome stability, and prevention of replication
catastrophe ......................................................................................................11 Structural mechanism for mediating RPA functions ......................................12 Regulation by protein-protein interactions ..............................................12 Regulation by post-translational modification ........................................13 Recent studies of RPA-dynamic binding ........................................................14 RPA binding to ssDNA is dynamic .........................................................14 High affinity binding of RPA is not sufficient for all its functions ................16 Repair-specific mutants ..................................................................................16 RPA-DNA interactions in replication and repair............................................17 CHAPTER 2 SINGLE MOLECULE ANALYSIS OF REPAIR-SPECIFIC RPA
MUTANTS REVEALE HIGH AFFINITY BINDING OF RPA IS
NEEDED FOR REPAIR ................................................................................23 Abstract ...........................................................................................................23 Introduction.....................................................................................................23 Materials and methods ....................................................................................27 Protein purification ..................................................................................27 DNA oligonucleotides .............................................................................28 Reaction conditions for the single-molecule assays ................................28 Single-molecule smTIRF.........................................................................29 smTIRF Data analysis .............................................................................29 Electrophoretic mobility assay and helix destabilizing assay .................30 Results.............................................................................................................31 ssDNA interaction with surface-tethered RPA........................................31 DNA-binding of surface-tethered RPA ...................................................33 Aro mutants have reduced binding to short ssDNA ................................34 Aro mutants are defective in forming “long-lived” complexes...............35 Aro mutant binding to partially duplex DNA structures .........................36 RPA and Aro mutants binding to Bubble DNA ......................................38 Discussion .......................................................................................................39 CHAPTER 3 SINGLE MOLECULE-BASED ANALYSIS OF
CONFORMATIONAL DYNAMICS OF THE RPA-SSDNA
COMPLEX .....................................................................................................76 Abstract ...........................................................................................................76 viii
Introduction.....................................................................................................77 Materials and methods ....................................................................................80 Constructs for expression of aldehyde tagged-DBD-F, DBD-A and
DBD-C .....................................................................................................80 DNA oligonucleotides .............................................................................81 Protein purification of aldehyde-tagged RPA .........................................81 Labeling aldehyde-tagged RPA ...............................................................81 Single-molecule smTIRF and reaction conditions for the singlemolecule assay .........................................................................................82 smTIRF Data analysis .............................................................................82 Results.............................................................................................................82 Fluorescence labeling of RPA for single-molecule imaging ...................82 RPA binds to different positions along the DNA ....................................84 RPA binds with 5’-3’ polarity and adopts a less dynamic and
condensed structure on binding ssDNA ..................................................85 The flexible DBD-F domain contributes to the FRET changes in
complex ...................................................................................................86 The evidence of microscopic dissociation within RPA-DNA
complex ...................................................................................................88 Discussion .......................................................................................................88 CHAPTER 4 DISSCUSION ............................................................................................111 Overview of findings ....................................................................................111 RPA-ssDNA interactions mediated by the conserved aromatic residues
are essential for cellular processes ................................................................112 The high affinity binding of DBD-A and DBD-B are essential for RPA
function .........................................................................................................114 Aromatic residues and polar residues play different roles in RPA
binding and functions. ..................................................................................116 Independent but coordinated RPA domains and nonequivalent function
of Aromatic residues .....................................................................................116 Regulation of RPA binding ..........................................................................117 The conformation and dynamics of RPA-DNA complex.............................118 Future directions for study of the aromatic mutants .....................................121 Summary .......................................................................................................122 APPENDIX I FUNCTION OF RPA4 IN CELLULAR DNA DAMAGE REPAIR
AND PROLIFERATION .............................................................................124 Abstract .........................................................................................................124 Introduction...................................................................................................124 Materials and Methods .................................................................................128 RNAi knockdown and replacement of RPA2 .......................................128 Flow Cytometry analysis .......................................................................129 Immunofluoresence analysis and DNA damage assays ........................129 Cell UV irradiation ................................................................................130 Chromatin-bound fractionation and immmunoblotting.........................130 Colon tissue immunohistochemistry .....................................................131 Lentiviral inducible Tet-off RPA expression constructs .......................132 Tet-off inducible system ........................................................................133 Making Tet-off cell line and double-stable Tet-off inducible cell
line .........................................................................................................133 Affinity purification of RPA4 antibody ................................................134 Results...........................................................................................................135 ix
RPA4 is unable to substitute for RPA2 to rescue cell cycle
progression ............................................................................................135 RPA4 can function in NER ...................................................................136 The NER specific-damage marker XPA is localized to chromatin
in response to 4NQO treatment .............................................................138 UV irradiation is used as an alternative way to induce NER ................139 Developing double-stable Tet-off inducible cell line with inducible
RPA4 expression ...................................................................................140 Transduction of HeLa Tet-off cells with inducible Lentiviral virus
showed Dox-regulated GFP-RPA2 and GFP-RPA4 expression ...........142 Selected colonies showed low inductivity and cells with inducible
RPA4 expression are negatively selected ..............................................143 Determine the distribution of RPA4 in normal and transformed
tissues ....................................................................................................143 aRPA showed altered interaction with slipped-DNA structure
within the CTG/CAG repeat ..................................................................145 Discussion .....................................................................................................146 APPENDIX II EXPRESSION AND ANALYSIS OF BIOTINYLATED RPA3 IN
MAMMALIAN CELLS ...............................................................................170 Introduction...................................................................................................170 Materials and Methods .................................................................................172 Construction of constructs to express biotinylated RPA in
mammalian cells ....................................................................................172 Biotinylated RPA expression in mammalian cells and purification ......172 Sorting phosphorylated RPA and non-phosphorylated RPA ................173 Results...........................................................................................................174 Construction of mammalian plasmid that express biotinylated
RPA3 in mammalian cells .....................................................................174 Detection of phosphorylated biotinylated RPA in cells ........................175 PhosphoRPA2 (S33) antibody is specific to phosphorylated RPA .......175 Phosphorylated RPA2 can be detected in single molecule
experiment .............................................................................................176 Discussion .....................................................................................................177 REFERENCES ................................................................................................................185 x
LIST OF TABLES
Table 2.1. Different length of ssDNA binding by RPA and Aro mutants. ........................64 Table 2.2. RFL and GAP binding by RPA and Aro mutants.............................................66 Table 2.3. Representative histograms of RPA and Aromatic mutants binding to
different lengths of ssDNA. ......................................................................................68 Table 2.4. Representative histograms of RPA and Aromatic mutants binding to
RFL and GAP DNA..................................................................................................73 Table 3.1. RPA-Cy5A and RPA-Cy5F bind to DNA substrates with the same high
affinity as RPA. The equilibrium constant is measured by smTIRF. .....................106 Table 3.2.The summary table for FRET distribution of RPA-Cy5A binding to
different labeled DNA substrates............................................................................107 Table 3.3. The summary table for FRET distribution of RPA-Cy5F binding to the
different labeled DNA substrates............................................................................109 xi
LIST OF FIGURES
Figure 1.1. Schematic of RPA subunits and structure. ......................................................19 Figure 1.2. Model of RPA binding involving transient dissociation of DBDs. .................20 Figure 1.3. Structural view of the high affinity DNA binding domains A and B. .............21 Figure 2.1. Biotin is covalently linked to the RPA3 subunit of RPA to surface tether
RPA in smTIRF. .......................................................................................................44 Figure 2.2. Surface-tethered RPA shows binding activity.................................................46 Figure 2.3.Mutation of aromatic residues affect DNA binding to short ssDNA. ..............48 Figure 2.4.The fraction of long-dwell complexes is dependent on length of ssDNA. ......50 Figure 2.5. Making partial duplex DNA structures. ..........................................................52 Figure 2.6. RPA and Aro mutant show high affinity toward RFL and GAP in
smTIRF. ....................................................................................................................54 Figure 2.7. RPA and Aro mutants show high affinity toward RFL with no helix
destabilization. ..........................................................................................................55 Figure 2.8. RPA and Aro mutants bind GAP DNA with high affinity with no helix
destabilization. ..........................................................................................................57 Figure 2.9. Aro mutants fail to stably associate with Bubble DNA and show
defective in melting activity. ....................................................................................59 Figure 2.10. RPA and Aro mutants do not melt DNAs containing 5’ or 3’ flaps..............61 Figure 2.11. Model of RPA multi-step dynamic binding. .................................................63 Figure 3.1.Site-specific modification of RPA at DBD-F and DBD-A. .............................93 Figure 3.2. Aldehyde modified RPA complex showed similar high binding affinity
to non-modified RPA complex. ................................................................................95 Figure 3.3. The FRET status of RPA-DNA complex with RPA-Cy5F and RPACy5A in smTIRF. .....................................................................................................96 Figure 3.4. The RPA-DNA complex remains fairly rigid for the duration of binding
events. .......................................................................................................................98 Figure 3.5. Representative dynamic complexes observed with RPA-Cy5A and
RPA-Cy5F binding to Cy3 3’ labeled dT35 and Cy3 5’ labeled dT66. .................100 Figure 3.6. DNA bound by RPA exhibits FRET dynamics during binding events. ........102 Figure 3.7. The proposed model of RPA-DNA complex based on observed FRET
states. ......................................................................................................................104 xii
Figure 4.1. Possible mechanisms of RPA binding...........................................................123 Figure AI.1. Human RPA2 homologue: RPA4. ..............................................................150 Figure AI.2. RPA4-expressing cells cannot progress through S-phase. ..........................151 Figure AI.3. Co-localization of RPA4 forms with γH2AX. ............................................153 Figure AI.4. RPA4 can partially mediate NER repair. ....................................................155 Figure AI.5. Using XPA as NER damage marker is unable detect recovery from
4NQO damage. .......................................................................................................157 Figure AI.6. Localization of chromatin-bound XPA after UV and 4NQO damage. .......159 Figure AI.7. Characterization of the TRE lentiviral vectors with inducible GFPRPA2 and GFP-RPA4 expression. .........................................................................161 Figure AI.8. Characterization of inducible lentivirus in HeLa Tet-off cells. ..................163 Figure AI.9.The induction of RPA4 is toxic to replicating cells. ....................................165 Figure AI.10. RPA4 staining pattern in colon crypt cryosection. ....................................166 Figure AI.11. Characterize the affinity purified anti-RPA4 antibody in western blot. ...167 Figure AI.12. RPA and aRPA melt slipped-DNA differently. ........................................169 Figure AII.1. Schematic representation of “single-molecule cell sorting”. .....................179 Figure AII.2. Detecting biotinylated RPA in mammalian cells. ......................................181 Figure AII.3. Characterizing phosphoRPA2 (S33) antibody in single molecule
experiment. .............................................................................................................183 xiii
LIST OF ABBREVIATIONS
4NQO, 4-nitroquinoline 1-oxide
6-4PPs, pyrimidine-(6-4)-pyrimidone photoproducts
Aro, Aromatic
ATM, ataxia telangiectasia mutated
ATR, ataxia telangiectasia and Rad3 related protein
ATRIP, ATR-interacting protein
BER, base excision repair
ChK1, checkpoint protein 1
ChK2, checkpoint protein 2
CPDs, cyclobutane pyrimidine dimers
Cpt, camptothecin
DAPI, 4’, 6-diamidino-2-phenylindole
DBD, DNA binding domain
DMEM, Dulbecco’s modified Eagle’s medium
DNA, deoxyribonucleic acid
DSB, double strand break
dsDNA, double-strand DNA
DTT, DL-Dithiothreitol
FACS, fluorescence activated cell sorting
FIV, Feline immunodeficiency virus
FRET, Förster resonance energy transfer
GFP, green fluorescent protein
HR, homology-mediated recombination
HU, hydroxyurea
MRN, MRE11-Rad50-Nbs1
MMEJ micro homology-mediated end joining
xiv
NER, nucleotide excision repair
nt, nucleotide
OB, oligonucleotide/oligosaccharide binding
PBS, phosphate buffered saline
PIK, phosphoinositide 3-kinase
RFL, Replication fork like
RNA, ribonucleic acid
RPA, replication protein A
siRNA, short interfering RNA
SSB, single-strand break
smTIRF, single molecule total internal reflection fluorescence
ssDNA, single-strand DNA
SV40, simian virus 40
Tag, T antigen
UV, ultraviolet
WH, winged helix
WT, wild type
XPA, xeroderma pigmentosum group A
XPF, xeroderma pigmentosum group F
xv
1
CHAPTER 1
INTRODUCTION
DNA exists primarily in duplex form that acts as a stable repository of genomic
information. However, in many cellular processes, including replication and DNA repair,
single-stranded DNA is exposed. The exposed ssDNA intermediates are less stable:
ssDNA is prone to chemical and enzymatic degradation, can be bound by inappropriate
enzymes, and can self-anneal to form secondary structures that hinder correct DNA
processing. To prevent these harmful processes, essential single-strand DNA binding
proteins (SSBs) sequester and facilitate the processing of single-stranded DNA in cells.
The SSB family of proteins is conserved in all organisms (1). SSB protein families share
limited sequence similarity and display diverse subunit organization, but all contain one
or more conserved oligonucleotide-binding (OB) fold domains (a five-stranded beta-sheet
coiled to form a closed beta-barrel) that mediate ssDNA binding (2). Originally, SSBs
were thought to function by coating single-stranded DNA (ssDNA) to prevent formation
of secondary structure and protect from degradation by nucleases. Later studies showed
that both bacterial and eukaryotic SSBs interact with specific protein partners to promote
efficient processing of single-stranded intermediates in DNA replication, repair and
recombination (3,4) and have multiple modes of interacting with ssDNA (5). Eukaryotes
use a heterotrimeric SSB known as “Replication Protein A” (RPA). RPA plays a central
role in many aspects of DNA metabolism, including being required for DNA replication,
repair and recombination (6). The characterization of RPA structure and functions thus
far indicate that RPA plays dynamic modulatory role in each of these processes through
DNA binding and specific interactions with other proteins. My studies provide a more
through understanding of RPA binding and function.
2
Eukaryotic single-strand DNA-binding proteins
The SSB families are characterized structurally by their oligosaccharide-binding
fold (OB), which is responsible for binding ssDNA (7,8). There are two core sub-groups
of SSB family; the simple SSB, which contain one OB-fold per polypeptide, and the
higher order SSBs, which contain multiple OBs. The human genome encodes both simple
and higher order SSBs. The simple SSBs are represented by human single-stranded DNA
binding protein 1 and protein 2 (hSSB1 and hSSB2) and the mitochondrial SSB (mtSSB),
while the higher order SSBs are represented by RPA (9). In addition, other proteins that
contain the OB-fold structure may also be considered members of the SSB family. For
example the TPP1-protection of telomeres (POT1), breast cancer susceptibility gene2
(BRCA2) are similar to the higher order of SSB (10). While containing only a single OBfold, the majority of simple SSBs assemble as higher order structures when they bind to
ssDNA. For example Escherichia coli SSB (ecSSB) is homotetramar and Deinococcus
radiodurans (drSSB) and Thermus acuqtics SSB are homodimers (4,11,12).
The majority of SSBs can be characterized based on their phylogenetic
distribution as being “bacterial”, “eukaryotic” and “archaeal”. While the bacterial SSBs
function as either homotetramers (e.g., ecSSB) or more rarely as homodimer (e.g.,
drSSB), both subtypes contain a total of four OB folds (13). Each bacterial SSB OB fold
is capable of binding to ssDNA in an arrangement in which the DNA wraps around the
outside of the oligomeric protein (14). The best-studied bacterial SSB is ecSSB.
Depending on the in vitro conditions and protein: ssDNA ratio, ecSSB tetramers can
utilize either two or four OB folds to bind ssDNA (14).
In eukaryotic cells, the major single-stranded DNA binding protein is Replication
Protein A (RPA). RPA was first identified and purified from human (HeLa) cell extracts
and was required to support simian virus 40 (SV40) DNA replication in vitro (15,16).
Analysis of RPA indicated that it is a heterotrimer composed of three subunits, RPA1,
RPA2 and RPA3 (15,16). Heterotrimeric homologues of the human RPA have been
3
isolated and identified in every eukaryotic organism examined, including Saccharomyces
cerevisiae, Schizosaccharomyces pombe, Xenopus laevis, Drosophila melanogaster as
well as in plants such as deepwater rice (17-20). Archaeal SSBs share qualities with both
bacterial SSB and eukaryotic RPA. For example Sulfolobus solfataricus has a bacterial
like SSB, while Methanococcus jannaschii has an SSB that is more similar to RPA
(21,22).
Besides RPA, human cells also have multiple other single-strand DNA binding
proteins (SSB) including mtSSB, hSSB1 and hSSB2. Eukaryotes encode a mitochondrial
SSB (mtSSB) within the mitochondrial genome (23). The eukaryotic mtSSB is a member
of the simple SSB sub-group that has a number of conserved residues in the N-terminus
that are shared with E.coli SSB (23). mtSSB plays a similar role to RPA in the nucleus,
except it functions specifically in the mitochondria (24). Recently, two nuclear members
of SSB family in human, named hSSB1 and hSSB2 have been identified (12). hSSB1 and
hSSB2 are much more closely related to the bacterial and archaeal simple SSB sub-group
than to RPA (7). They have one OB fold and a C-terminal tail that is predicted to interact
with proteins. hSSB1 and hSSB2 are found to have a role in repair of double-strand DNA
breaks by homologous recombination and ataxia telangiectasia-mutated (ATM)-mediated
checkpoint pathways (12,25). In contrast to RPA, hSSB1 is not required for DNA
replication.
RPA structure
RPA is composed of three subunits of 70, 32, and 14 kDa (RPA1, RPA2, and
RPA3, respectively) (3,6,8) (Figure1.1). Each of the RPA subunits contains one or more
OB folds commonly referred as DNA-binding domains (DBD) (26). DBDs are
designated with letters A-F (Figure 1A). The three subunits of RPA form a very stable
complex with one DBD in each subunit interacting to form the trimerization core (DBDC, -D, -E) (27). All the other parts of RPA extend from the trimerization domain on
4
flexible protein linkers. The flexible, often long, unstructured linkers allow the other
domains in RPA to rotate independently and to adopt a variety of conformations (28).
RPA1 contains four DBDs (Figure 1A). DBD-F at the N-terminus of RPA1 (also
known as the N-terminal domain) can interact with DNA but is thought to primarily
function as a regulatory, protein-interaction domain (29-31). DBD-A, -B, and -C are
primarily required for binding ssDNA but also interact with protein partners. DBD-C is
part of the trimerization core (27). DBD-A has 5-10 fold higher affinity for ssDNA
(Kd=1.7 µM) than the other DNA binding domains (DBD-B Kd=16 µM) (32,33). In
addition the short linker that connects DBD-A and DBD-B allows these two domains to
act as a high affinity binding site with an affinity ~100 folder higher than isolated
domains (33).
RPA2 is composed of two structured domains: a central DNA binding domain
(DBD-D) and a C-terminal winged helix domain (wh) (Figure 1A). DBD-D interacts
with ssDNA and is part of the trimerization core while the winged helix domain is
primarily involved in protein interactions. In addition, the N-terminal domain of RPA2
(called the phosphorylation domain; Pd) is unstructured and becomes multiply
phosphorylated after DNA damage (34,35). RPA3 is composed exclusively of an OBfold (DBD-E) that interacts weakly with DNA (36) and is part of the trimerization core
(37).
RPA4 and Alternative RPA complex
RPA is a highly conserved complex and all eukaryotes contain homologs with
three subunits (6). Some organisms, such as seed plants (rice, Arabidopsis thalianan) and
some protists, contain multiple RPA subunits genes that form multiple RPA complexes
(38). In rice, for example, evidence has shown that multiple RPA complexes perform
different cellular functions (38-40). Human cells have a single additional subunit called
RPA4 which is a homolog of RPA2 subunit. RPA4 was initially identified as one of the
5
proteins that interacts with RPA1 from a HeLa cell cDNA library (41). The genomic
analysis of RPA4 sequence showed that RPA4 is an intronless gene on the X
chromosome. RPA4 homologs with complete coding sequence are only found in
primates: human, chimpanzee, organgutan, rhesus monkey and marmoset. Horse also
contains a complete coding sequence (42).
RPA4 has 63% amino acid similarity to RPA2 sequence and also appears to have
similar domain organization, with the putative phosphorylation domain at N terminus, the
putative DBD (DBD-G) and the putative winged helix domain (wh) (42). RPA4 can
substitute for RPA2 to form an alternative RPA complex (aRPA) (41,43). Biochemical
analysis indicated that aRPA forms with similar efficiency to canonical RPA (43). RPA4
mRNA level has been detected in normal human tissues and has reduced expression in
cancers (44). RPA4 is not expressed at a significant level in cultured cell lines suggesting
that RPA4 is down regulated in transformed cells (44).
aRPA exhibits high binding affinity to ssDNA similar to RPA, but it does not
support SV40 replication (43). It was later found that aRPA has weaken interaction with
DNA polymerase α and does not support the primer synthesis by polymerase α during
initiation steps of replication (45). However, aRPA can still support processive DNA
synthesis by DNA polymerase δ in the presence of RFC and PCNA (45). In human cells,
the expression of exogenous RPA4 does not support S phase progression and causes cell
cycle arrest in G2/M (42). On the other hand, RPA4 is able to support checkpoint
activation and expression of RPA4 can suppress the cell death caused by RPA2 depletion
(42). In addition, RPA4 is able to localize to the repair foci after DNA damage (42). In a
reconstituted in vitro assay, aRPA can support Rad51-mediated strand exchange in
homologous recombination and support the dual incision/excision reaction of nucleotide
excision repair (44). These initial studies on RPA4 suggested that aRPA has different
functions than RPA. aRPA can support DNA repair but prevents proliferation. It is
proposed that aRPA is important for maintaining the genome integrity in differentiated
6
cells. The role of RPA4 in DNA repair and proliferation is further investigated in my
thesis.
DNA binding modes of RPA
RPA binds single-stranded DNA with high affinity; however, the flexibility of the
RPA complex has made it difficult to study RPA binding to DNA structurally. It was
only in 2012 that the structure of RPA from Ustalago maydis stably bound to singlestranded DNA was determined (Figure 1B; (46)). This structure and other biochemical
analysis show that four DBDs in RPA (A-D) form a stable complex with ~30 nt of
ssDNA. RPA binds ssDNA directionally with DBD-A at the 5’ end and DBD-D at the 3’
end of the complex (46-48).
Each of the DNA-binding domains interacts with approximately 4-6 nucleotides
of single-stranded DNA (46,49). Early reports suggested that there were multiple modes
of RPA binding, including an unstable 8 nt binding mode (50) and a stable 30 nt binding
mode (50,51). The 8-10 nt binding mode has only been observed after crosslinking (50)
or when RPA interacts with very short oligonucleotides (52). In contrast, detailed
thermodynamic analysis of RPA binding identified only 18-20 and 28-30 nt binding
modes (53,54). It seems likely that these two stable modes of binding represent 3 and 4
DBDs interacting with DNA (Figure 1A). So the current paradigm is that RPA binds
single-stranded DNA by sequentially engaging DNA-binding domains, hence creating
more stable complexes as more DNA is bound. This simple model accounts for many of
the properties of RPA binding: the affinity of RPA decreases as DNA length decreases
(51), and RPA adopts different conformations depending on the length of DNA bound
(52). However, the recent studies of RPA, discussed below, suggest that the interaction of
individual DNA-binding domains is not sequential, and that RPA-binding is better
described by a dynamic model.
7
Cellular functions of RPA
RPA in DNA replication
RPA is first identified as the essential component for simian virus 40 DNA
replication (15,16). In replication, there is extensive unwinding and ssDNA intermediates
with up to hundreds of nucleotides are exposed. RPA coats the ssDNA intermediates and
interacts with multiple replication proteins at the replication fork (3,6). RPA is required
for both initiation and elongation steps of replication (16,55). RPA coordinates the
polymerase switching on the lagging strand from low-fidelity DNA polymerase α, which
initiates synthesis of Okazaki fragments, to the high-fidelity DNA polymerase δ (56).
This process is mediated by RPA sequentially binding to and releasing polymerase α,
replication protein C, and polymerase δ. During maturation of the lagging strand, the
RNA containing Okazaki fragment is displaced because of DNA synthesis catalyzed by
polymerase δ (55). RPA binds to this nascent flap and coordinates the endonucleases
Fen1 and DNA replication Dna2 which remove the RNA primer of Okazaki fragment
(57).
RPA in DNA repair
RPA has also has important roles in DNA repair (58). Nucleotide excision repair
(NER) is the major excision mechanism to remove the helix-distorting lesions caused by
ultraviolet light (UV) and other bulky adducts. The recognition of the lesion leads to
removal of a short ssDNA segment containing the lesion. RPA participates in damage
recognition, excision and re-synthesis reactions of NER (58). The assembly of the initial
repair complexes in NER requires RPA to maintain local separation of the two strands
after DNA recognition (59). The joint recognition of a DNA damage site by RPA and
XPA is needed for recruitment of excision endonuclease at the site (60). Also, the
polarity of RPA bound to the undamaged strand spatially coordinates endonuclease XPG
on the 3’ end and ERCCI-XPF on the 5’ end for the excision reaction (47). The excision
8
of the damaged strand leaves RPA bound to a gapped DNA where it coordinates the
assembly of RFC, PCNA and polymerase δ to repair the gap (56,61).
Double-strand breaks (DSBs) are deleterious to cells as both strands of DNA
duplex are broken. DSBs are highly toxic and can cause genome rearrangements and cell
death. Two mechanistically distinct pathways have evolved to repair DSBs: homologous
recombination (HR) and non-homologous end joining (NHEJ). NHEJ involves the direct
ligation of DSBs ends. Canonical NHEJ is defined as being dependent on Ku and ligase
IV and can occur with high fidelity or be associated with small deletions or insertions at
the junctions. HR is the major, error-free repair pathway for repairing DSBs and has been
studied extensively in budding yeast. In HR, the 5’ strand of the double strand break is
resected to produce a 3’ overhang (62,63). It’s been proposed that RPA might have a role
in making the choice between NHEJ and HR pathways. It was found that Ku and RPA
compete for binding to ends with ssDNA (64). Ku blocks resection while RPA
participates in resection and then coats the resulting 3’ overhang. In HR, subsequent
protein interactions with recombination mediators result in the loading of Rad51 that, in
turn, catalyzes recombination (65). Rad51 forms filament with ssDNA and mediates
DNA-strand exchange. The Rad51-ssDNA filament exchange is greatly enhanced in the
presence of RPA, which removes the DNA secondary structure from the ssDNA tails
(66). The Rad51-ssDNA nucleofilament searches for homologous DNA sequence in the
genome to form a displacement loop or D-loop, in which DNA synthesis is initiated to
replace the resected DNA surrounding the former break site. RPA is known to bind the
displaced strand in the D-loop and stabilize D-loop formation (67). RPA also facilitates
the recombination-mediated synthesis by increasing the efficiency of primer utilization
and prevent polymerase stalling (68). Finally, the D-loop structure is resolved either
through synthesis-dependent strand annealing (SDSA) or through migrating double
Holiday junction (dHJ) intermediates that are cleaved (crossover) or dissolved (non-
9
crossover). RPA is needed to sequester the ssDNA intermediate during dHJ resolution
(69). HR repair is error-free with no loss of genetic material (70).
After resection of a DSB, a second (alternative) pathway, micro-homologymediated end joining (MMEJ), competes with HR to fix the break. In MMEJ, short
homologous single-stranded regions (between eight and twenty nucleotides in length) on
the 3’ overhangs anneal, the DNA flaps are excised, gaps filled in and the DNA ligated
(71). This pathway is error-prone; any DNA sequences between the regions of microhomology are lost during repair. A recent paper by Deng and co-workers showed that
RPA binding influences the choice of the pathway used in DSB repair, inhibiting MMEJ
and stimulating HR (72).
RPA and DNA damage response
Respond to and repair of DNA damage is crucial for cell survival and genome
maintenance. DNA damage response (DDR) is a signal-transduction pathway that
coordinates cell-cycle transitions, DNA replication, DNA repair and apoptosis using
cellular checkpoints (73). When a checkpoint is activated, cell cycle progression is halted
until the DNA damage is repaired.
The major regulators of the DNA damage response are the PI3K-related protein
kinases (PIKKs) including ATM and ATR (73). ATM and ATR are both large kinases
with a strong preference for phosphorylating Ser or Thr residues that are followed by Gln
(73). They both target an overlapping set of substrates that promote cell-cycle arrest and
DNA repair. However, ATM is primarily activated by DNA DSBs caused by ionizing
radiation (IR) or radiomimetic drugs, whereas ATR responds to replicative stress and
other forms of DNA damage, such as that caused by ultraviolet light (UV) (74). Also,
ATR and ATM are recruited to sites of DNA damage by different factors. ATM is
recruited to DSBs by the Mre11-Rad50-Nbs1 (MRN) complex, whereas ATR is recruited
by ATR-interacting protein (ATRIP) binding to RPA-coated ssDNA that forms at stalled
10
replication forks or after processing of DNA damage (75-77). After being recruited to
sites of DNA damage, ATM and ATR phosphorylate a number of proteins, including the
protein kinases, ChK1 and ChK2, which target other proteins to induce cell-cycle arrest
and DNA repair (78). As another member of PIKK kinase family, DNA-PK plays a key
role in non-homologous end joining (NHEJ) by recognizing DSBs, initiating NHEJ
repair and assembling the repair machinery. DNA-PK also phosphorylates proteins
involved in DDR, such as H2AX, RPA, p53, XRCC4, Ku70 and Ku80.
RPA-covered ssDNA is needed to activate ATR-Chk1 signaling pathway during
replication stress. The recruitment of ATR to sites of DNA is through a physical
interaction between ATR-interacting protein (ATRIP) and RPA (29). RPA coated ssDNA
is sufficient for localizing the ATR-ATRIP complex. However, ATR kinase activation
still requires a co-localization of the ATR-ATRIP with RAD9-HUS1-RAD1 (9-1-1)
complex (79). RPA also directs the loading of 9-1-1 complexes at the primer-template
junctions with a preference for a 5’ recessed end (80,81). The 9-1-1 complexes
concentrate the ATR activator, TopBP1, to sites of DNA damage or replication stress
(82). TopBP1 stimulate ATR kinase activity, which leads to phosphorylation of
downstream proteins (83). The other proteins interact with RPA during DDR including
tumor suppressors, p53, BRCA1, BRAC2 (29,84-86). RPA also interacts with Mre11,
which acts as a double-strand break sensor upstream of ATM by binding to the exposed
dsDNA ends (87).
RPA interacts with ssDNA intermediates in different
cellular pathways
As discussed above, RPA is required for cellular replication, repair, and
recombination (3,6,35). RPA also functions in coordination of the cellular response to
DNA damage and is required for activation of cellular checkpoints (29,75). The common
feature of all pathways requiring RPA is that each has ssDNA intermediates; however,
11
different pathways have intermediates that differ in length, form of adjacent DNA (e.g.
DNA end vs. duplex DNA) and associated proteins. This means that RPA must be able to
recognize and facilitate the selective processing of diverse ssDNA intermediates.
RPA is an abundant protein in cells and binds to ssDNA with subnanomolar (nM)
affinity (51,53). Thus, any single-stranded region formed in genomic DNA is
immediately bound by RPA. The resulting RPA-ssDNA complex then interacts with
protein partners to coordinate the processing of the ssDNA (3,35). The mechanism by
which RPA is able to direct different ssDNA intermediates to different pathways and
coordinate replication, recombination and repair is not understood. In my thesis, I will
study how RPA interact with ssDNA intermediates found at sites of DNA damage and
replication. In Chapter 2, I characterized the interactions of RPA and repair-specific
mutants with different length ssDNAs and partial duplex DNA structures like those found
in DNA repair to determine RPA-DNA interactions required for DNA repair.
Cellular RPA levels, genome stability, and prevention of
replication catastrophe
Loss of any of the subunits of RPA is lethal (31,88) and non-lethal mutations in
RPA can cause DNA repair defects and genome instability (89-91). It is also clear, that
while under normal circumstances the cellular pool of RPA is sufficient for all required
DNA transactions, reduction in the cellular level of RPA is deleterious. In mice,
haploinsufficiency of RPA causes a high rate of lymphoid tumors and a shortened
lifespan (92,93). In humans, heterozygous deletion or duplication of the RPA1 gene lead
to changes in protein levels that cause defects in the cellular DNA damage response
(94,95).
It has been shown that RPA is important to protect the excess of ssDNA during or
after replication stress (96). The RPA level directly affects cell tolerance to long and
unstable ssDNA in the stalled replication fork by preventing fork breakage (96). Under
12
replication stress, the number of ssDNA regions is dramatically increased due to fork
stalling. RPA is recruited to these sites where it helps stabilize the stalled forks and
signal for ATR activation to stop the cell cycle and ongoing replication (97). Without the
ATR checkpoint activation, new replication origin firing will continue and keep
generating new ssDNA. If the level of ssDNA becomes high enough, there is insufficient
RPA (called “RPA exhaustion”) to protect ssDNA and rapid conversion of singlestranded DNA to double-strand breaks, which cause replication catastrophe and cell
death.
Structural mechanism for mediating RPA functions
Dynamic binding of RPA is needed to function in different DNA pathways, where
RPA plays a role in regulating and coordinating assembly and disassembly of DNAprocessing factors on ssDNA. As a key hub protein, RPA interacts with many other DNA
processing proteins and is subjected to post-translational modification. It’s been proposed
the dynamic binding of RPA is mediated by modulating RPA’s structure though proteinprotein interactions and post-translational modification (3).
Regulation by protein-protein interactions
RPA interacts with a number of protein partners in DNA replication repair and
recombination (3). These interactions are essential for RPA function in these pathways.
RPA has multiple sites for protein interactions (29,98-103). With so many protein
partners, proteins from different pathways need to compete to bind RPA. The competition
for the binding site of RPA is likely to regulate pathway choices and ensure the ordered
progression down a pathway. The good examples include that RPA coordinate
polymerase switching and nucleotide excision repair (35,56). Support for the role of
competition in pathway choices was shown in a study of the winged helix domain of
RPA2, which was shown to bind a common motif from XPA, Rad52 and UNG2, which
are involved in NER, base excision, and recombination repair, respectively (99).
13
Most RPA-protein interactions involve parts of the protein outside the DNAbinding sites in DBD-A-D. This suggests a model in which RPA-protein interactions may
be directly modulating DNA-binding by regulating individual DBDs or altering the
conformation of RPA. For instance, the homologous recombination mediators Rad52 was
found to stimulate the ssDNA binding affinity of RPA by a factor of 5, and this effect has
been attributed to the increased binding to the DBD-D domains (104). In another case,
interaction of the SV40 T-antigen helicase with DBD-A and DBD-B stimulate RPA
binding affinity (101). It has been also been previously suggested that protein
interactions with DBD-F (at the N-terminal domain of RPA1) or with the winged helix
domain of RPA2 modulate checkpoint activation and replication, respectively
(3,29,105,106).
Regulation by post-translational modification
RPA is post-translationally modified in cells. RPA phosphorylation and
SUMOylation are thought to help regulate the cellular recovery to DNA damage. There
are multiple phosphorylation sites at the N-terminus of RPA2 and several other less
characterized phosphorylation sites on the other subunits (35,107). In undamaged cells,
RPA becomes phosphorylated at the G1/S phase transition and is subsequently
dephosphorylated after mitosis (108). In addition to the cell-cycle-dependent
phosphorylation events, RPA is hyperphosphorylated in cells with DNA damage (34,35).
DNA-damaged induced RPA phosphorylation depends on the activity of three DNA
damage repair kinases of the phosphoinositide 3-kinase (PI3K)-like protein kinase
(PIKK) family: ATM, ATR and DNA-PK (109). Crosstalk between these DNA damage
repair kinases during RPA phosphorylation is complicated and different sites are
phosphorylated in response to particular types of DNA damage (34,35). RPA
phosphorylation is important for recovery from DNA damage and replication stress in S
phase (97,110-112), and genotoxic stress in mitosis (112,113). RPA phosphorylation has
14
also been suggested to regulate homologous recombination after replication arrest
(112,114,115). It is known that phosphorylation modulates both protein and DNA
interactions (116-118) but how these changes regulate the cellular DNA damage response
remain poorly understood.
RPA is also SUMOylated at lysine residues in the C-terminal domain of RPA1 in
response to DNA damage (119). Mutation of the sites of SUMOylation (K449 and
K577) causes cells to be more sensitive to DNA damage (119). In addition,
SUMOylation of RPA appears to stimulate loading of RAD51 at sites of DNA damage
(119). Again, the mechanism of regulation of RPA by SUMOylation is not known. It
seems likely that post-translational modifications could be regulating RPA function by
affecting the conformations or dynamics of RPA-ssDNA complexes.
Recent studies of RPA-dynamic binding
Several recent studies have dramatically changed our understanding of how RPA
interacts with ssDNA and its role in cells. First analysis of DNA binding mutants has
shown that affinity for ssDNA (as measured by binding to oligonucleotides) does not
directly correlate with RPA functions (120). In addition, single molecule studies have
shown that RPA binding to ssDNA is the result of having multiple domains interacting
dynamically with DNA and suggest that these dynamic interactions reduce processing by
error-prone pathways while promoting recombination repair of double strand breaks
(DSBs) in yeast (72). This suggests that the RPA-ssDNA complex plays an active role in
determining how different ssDNA intermediates are channeled into selected pathways.
RPA binding to ssDNA is dynamic
RPA binds to DNA very tightly and also needs to be replaced by other proteins to
gain access to the substrate. The high affinity RPA binding is the result of having
multiple contacts by tethering multiple DNA-binding domains together, yet each domain
exhibits different binding affinity (33,46,121). Proteins like RPA that are composed of
15
multiple, and flexible attached domains, can undergo intra and inter-domain
arrangements (46,52). This property might allow RPA to interact optimally with diverse
DNA substrates present during DNA processing. Recently studies have shown that RPA
interactions with DNA are highly dynamic and suggest that microscopic interactions of
individual DNA-binding domains may contribute significantly to RPA function. Gibb
and coworkers used single molecule imaging of yeast RPA on single-strand DNA
curtains to examine RPA binding at a molecular level (122). “DNA curtains” are created
by aligning thousands of lipid-tethered long DNA molecules on the surface of a
microfluidic sample chamber where they can be visualized by smTIRFM (123). When
RPA bound to ssDNA curtains, it formed a stable complex that very rarely dissociated.
However if there was free RPA or other ssDNA-binding proteins present, bound RPA
was found to rapidly exchange. This suggests that in the presence of other ssDNAbinding proteins, RPA can be rapidly removed from the DNA. The second study, by
Nguyen and coworkers, analyzed individual molecules of RPA bound to single-stranded
DNA (54). This analysis showed that human RPA could rapidly diffuse along singlestranded DNA without dissociating. The rate of diffusion of RPA is ~ 5000 nt2 sec-1 at
37°C. Furthermore, these studies showed that this rapid diffusion was productive and
promoted the destabilization of small adjacent DNA hairpins. Both studies suggest that
the DNA binding properties of RPA are the result of having multiple DBDs linked in a
flexible structure interacting with ssDNA. The microscopic affinity of each DBD is
modest but together they give the complex very high affinity (a subnanomolar
macroscopic dissociation constant.) Current models explain these finding by suggesting
that RPA binding to ssDNA involves transient dissociation of single-DNA binding
domains (Figure 1.2).
16
High affinity binding of RPA is not sufficient for all its
functions
The dynamic model for RPA binding to ssDNA may help explain a consistent
mystery regarding RPA function. This mystery is that RPA affinity for single-stranded
oligonucleotides does not directly correlate with RPA function. Mutational analysis of
the DNA-binding sites in RPA has generated forms with reduced ssDNA-binding
affinity. In this class, some mutations that reduce the affinity of the complex by two
orders of magnitude are fully functional in cells, while other mutations that have a higher
affinity for oligonucleotides are partially or completely inactive (31). In particular,
mutation of conserved aromatic residues in the DNA binding sites of DBD-A or -B cause
a separation-of-function phenotype: the mutants support DNA replication but are
defective in DNA repair (120). These results suggest that high affinity for ssDNA is not
sufficient for the full function of RPA and that replication and repair require different
RPA-DNA interactions. It now seems likely that the observed loss in activity arises
because of the mutants are affecting the kinetics or microscopic interactions of individual
DBDs in the dynamic RPA-ssDNA complex. These data suggest that altering the
dynamics in the RPA-ssDNA complex affects activity without a comparable affect on the
macroscopic affinity constant.
Repair-specific mutants
RPA1 subunit mediates high affinity binding to ssDNA. RPA-DNA interface
contains a series of polar residues and four conserved aromatics residue (49). We used a
combination of biochemical analysis in vitro and knockdown-replacement studies in vivo
to characterize the contribution of aromatic residues in RPA function. These four
conserved aromatic residues in the high-affinity binding domain of RPA1 are F238 and
F269 in DBD-A, and W361 and F386 in DBD-B of RPA1 (Figure 1.3 A and B). Two
mutant forms of RPA, AroA and AroB have double aromatic mutations in DBD-A
17
(AroA-F238A, F269A), and DBD-B (AroB-W361A, F386A), respectively (Figure 1.3
C). Two other aromatic mutants, Aro1 (F238A, W361A) and Aro2 (F269A, F386A),
have one aromatic residue in each DBD-A and DBD-B (Figure 1.3 C). Aro1 is a null
mutant that has undetectable DNA binding activity.
Mutation of these aromatic residues results in separation-of-function phenotype.
Cells expressing the aromatic mutants supported DNA replication, had normal
checkpoint activation after DNA damage but were defective in DNA repair (120).
Biochemical characterization revealed that mutations of aromatic residues altered the
stability of the RPA-DNA complex and decreased the affinity for the short ssDNA (<20
nt) (120). My goal was to determine the repair-specific role(s) of aromatic residues and
reveal the RPA-DNA interactions required for repair but not replication.
RPA-DNA interactions in replication and repair
It now appears that dynamic RPA interactions are critical for correct processing of
different single-stranded DNA intermediates in the cell. However, it is still not clear how
the different domains in RPA contribute to these properties. In addition, the mechanism
by which mutations that affect the binding of one DBD specifically disrupt DNA repair
without affecting DNA replication is not known. More detailed analysis of RPA-DNA
interactions, particularly the dynamics of the RPA-DNA complex, is needed to
understand the contributions of individual DBDs to RPA binding and functions.
In this thesis, Chapter 2 investigated how aromatic residue-mediated interactions
are important for RPA to function in DNA repair using single molecule analysis. These
studies have been completed and prepared for publication. In Chapter 3, I described data
on single molecule analysis to define the dynamics of the RPA-ssDNA complex.
Appendix I is focused on studies to define the role of the alternative RPA complex in
DNA repair pathways in cells. In this Appendix, I described the studies to establish
techniques and optimization of conditions for studying the role of aRPA in proliferation
18
and repair in cultured cells. The strong negative effects of expressing RPA4 in cells
limited the progress of these studies. In Appendix II, a selection of data describing the
application of a single molecule sorting method to examine at the function of in vivo
post-translational modified RPA2 is included. Taken together, theses studies contribute to
our understanding of the molecular interactions of RPA with ssDNA intermediates in
different DNA metabolic pathways and expand the field’s knowledge of how RPA-DNA
interactions contribute differently to DNA repair and replication.
19
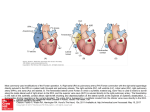
Figure 1.1. Schematic of RPA subunits and structure.
(A) The position of aromatic residues that are mutated in Aromatic mutants are
indicated in RPA1 subunits. (DBD, DNA binding domain) WH, winged helix. Line,
unstructured linkers. (B) Structure of Ustilago maydis RPA binding to DNA (Cyan) (46).
A
RPA2!
RPA1!
DBD-F!
DBD-A!
DBD-B!
F238 F269!
W361 F386!
Core DNA binding domains!
DBD-C!
DBD-D!
2° binding domains!
RPA3!
DBD-E!
B
wh!
20
Figure 1.2. Model of RPA binding involving transient dissociation of DBDs.
In this model, stable macroscopic binding of RPA to ssDNA includes constant
microscopic dissociation, rebinding of individual and subset of the DBDs. The rapid
binding and dissociation allows the complex to rearrange and diffuse along ssDNA
without dissociation. In the presence of other single-stranded DNA binding proteins
(SSB), RPA is displaced.
21
Figure 1.3. Structural view of the high affinity DNA binding domains A and B.
Tandem domain A and B are shown in green with conserved aromatic residues
shown in pink. DNA is shown in blue. Both front view (A) and side view (B) are shown.
Modeling is based on the crystal structure (PDB: 1JMC) and performed with PyMOL.
(C) List of Aro mutants with aromatic residues mutated.
22
A
B
C
23
CHAPTER 2
SINGLE MOLECULE ANALYSIS OF REPAIR-SPECIFIC RPA
MUTANTS REVEALE HIGH AFFINITY BINDING OF RPA IS
NEEDED FOR REPAIR
Abstract
RPA, the major eukaryotic single-stranded DNA (ssDNA) binding protein, is
essential for replication, repair, recombination, and cell cycle progression. Defects in
RPA activities lead to genome instability, a major contributor to the development of
cancer and other disease. ssDNA binding activity is mainly mediated by two domains in
the large subunit of RPA (RPA1). These ssDNA interactions are mediated by a
combination of polar residues and four conserved aromatic residues. Mutation of these
aromatic residues results in separation-of-function phenotype. Cells expressing the
aromatic mutants supported DNA replication, but were defective in DNA repair. We used
both ensemble and single-molecule fluorescence approach to determine the affinity and
kinetics of binding of aromatic mutants to different substrates including single strand
intermediates found at sites of damage and replication. Mutation of the aromatic residues
altered the stability of the RPA-DNA complex and decreased the affinity for short
ssDNA regions. Our results show that DNA replication and DNA repair require different
RPA-DNA interactions and that functions in repair depend on the high affinity DNAbinding domains of RPA1. These studies contribute to our understanding of how human
cells maintain genome integrity.
Introduction
Efficient repair of DNA lesions and faithful replication are essential to maintain
genome integrity. The major single-stranded DNA (ssDNA) binding protein in human
cells, Replication protein A (RPA) is essential for DNA replication, repair and
24
recombination (6,35,124). RPA also is required for checkpoint activation (29,75,125127). RPA functions by binding to ssDNA where it prevents formation of secondary
structures and nuclease digestion (124). RPA also interacts with protein partners and
coordinates assembly of the complexes that synthesize and repair ssDNA (3,35,124,128).
RPA participates in both initiation and elongation steps of replication. In
initiation, RPA promotes the recruitment of proteins to the origin complex and during
elongation it promotes loading of DNA polymerases α, δ and ε, coordinates the
polymerase switch on lagging strand and processing of Okazaki fragments (55,57,129131).
RPA is also required for most DNA repair pathways including nucleotide excision
repair, recombination repair, and mismatch repair (132-134). In nucleotide excision
repair (NER) RPA interacts with XPA to stabilize the open complex after damagerecognition, helps position the nuclease for dual incision and fill in the gap after excision
(58,135,136). During repair of double-strand breaks (DSBs) by homologous
recombination (HR), RPA is involved in end resection and loading of Rad51 to facilitate
Rad51-mediated strand exchange and subsequent annealing (62,63,70). RPA binding also
down-regulates spontaneous annealing to prevent an error-prone DSB repair pathway,
microhomology-mediated end joining (MMHJ), and to favor the HR repair pathway (72).
RPA is composed of three subunits, RPA1, RPA2 and RPA3 (6). Each RPA
subunit contains one or more oligonucleotide binding (OB) folds that are referred as
DNA-binding domains (DBDs) (26). RPA1 consists of four OBs (DBD-F and A-C)
(30,49,121), connected by flexible, unstructured linkers. RPA2 contains two structural
domains, one OB fold (DBD-D) and a winged helix (WH) domain. RPA3 is composed
exclusively of a single OB fold, DBD-E (36). The three subunits of RPA form a stable
complex with one DBD from each subunit interacting to form a trimerization core (27).
Other domains extend from the trimerization core on the flexible linkers (28). Structural
studies have shown that four DBDs interact with ssDNA to form a stable complex with
25
~30 nt of ssDNA (46). RPA binds to ssDNA directionally, with domains A through D
binding from the 5’- to the 3’-end of a given sequence (46-48).
RPA binds ssDNA with low specificity and high affinity (Ka~1010 M-1), with an
occluded binding site of ~30 nt (46,51,54). DNA binding domain A (DBD-A) and DNA
binding domain B (DBD-B) from RPA1 have the highest affinity for ssDNA and form
the primary binding site in RPA (32,33,137). Individual DBD-A and DBD-B can bind
ssDNA with Kd of ~2 µM and 20 µM, respectively (33). The complex of DBD-A and
DBD-B connected by a short linker increased the binding affinity ~100-fold (Kd ~50 nM)
as compared to the single DBD (32,33). DBD-C of RPA1 subunit and DBD-D of RPA2
subunit are secondary binding domains with weaker binding affinity (27,32,37,138). The
affinity of RPA to the bound ssDNA differs depending on the length of the ssDNA and
the number of DBDs involved (32,50,51,139). The high affinity binding of RPA engages
all four DBDs (DBD-A, DBD-B, DBD-C and DBD-D) to form the stable RPA-DNA
complex (27,46,50,52,140).
RPA does more than tightly bind to ssDNA, RPA also needs to recruit and be
displaced by proteins that process the ssDNA. Recent studies suggest that this process is
enhanced by the dynamic interactions between RPA and DNA (54,122). RPA binds
ssDNA tightly without dissociation but can be exchanged in the presence of free RPA
and other ssDNA-binding proteins (122). These studies suggested that RPA could be
rapidly removed from ssDNA in the presence of other ssDNA-binding proteins. It was
proposed that this exchange was caused by microscopic dissociation of individual
domains of RPA, which make small ssDNA regions for other ssDNA-binding proteins to
bind and facilitate RPA dissociation (122,124). In another single molecule study, the
activity of individual RPA on bound DNA was analyzed. This study showed that RPA is
able to diffuse along the ssDNA after binding, with a rate of diffusion (~5000 nt2seoncds1
) (54). Diffusion of RPA contributes to melting of the secondary DNA structure. Both
26
studies suggested that dynamic bindings of RPA are the result of multiple DBDs linked in
a flexible structure interacting with ssDNA (124).
The high affinity domains DBD-A and DBD-B interact with ssDNA by means of
polar and aromatic residues, including four aromatic residues (49). The four aromatic
residues, phe-238 and phe-269 in DBD A and trp-361 and phe-386 in DBD B, are highly
conserved in eukaryotes (120). These aromatic residues mediate RPA-ssDNA contacts
through base stacking (49). To study the functions of conserved aromatic residues, we
mutated pairs of these residues to alanine. Mutation of individual aromatic residues had
minimal effects on binding affinity (31,137). In contrast, when pairs of aromatic residues
were mutated there were significant defects in DNA binding and function. When both
aromatic residues in either DBD-A or DBD-B were mutated, binding affinity was
decreased by an order of magnitude (32,137). These two mutant forms were called AroA
(F238A, F269A) and AroB (W361A, F386A). The other two double aromatic residue
mutants, Aro1 (F238A, W361A) and Aro2 (F269A, F386A), had one residue in each
domain mutated. Aro1 was found to be a null mutant and had undetectable DNA-binding
activity while Aro2 had a modest affect on binding (31,137). When the functions of
AroA, AroB and Aro2 were tested in cells, they were found to have a separation-offunction phenotype: they were defective in DNA repair but still supported replication
(31,120). These studies demonstrated that these aromatic residues are essential for DNA
repair. These mutations are in the DNA binding sites in DBD-A and -B and have been
found to have no defects in protein interactions. We concluded that DNA replication and
repair require different RPA-DNA interactions. Ensemble biochemical studies suggested
that the binding activity of aromatic mutants to short ssDNA is altered. However, the
DNA binding defect(s) that disrupts DNA repair is still unknown.
To gain a better understanding of the molecular defects responsible for the loss of
activity in DNA repair, we analyzed the DNA interactions of the Aro mutants using
single molecule total internal reflection fluorescence microscopy (smTIRFM). Our
27
studies show that the interactions of the Aro mutants with linear and partially duplex
DNA structures 20 nt or longer are similar to wild-type RPA. However, the Aro mutants
cannot form complexes with oligonucleotides 15 nt in length. In addition, our kinetic
analysis suggests that wild-type RPA has multiple states when binding to ssDNA: a fastand a slow-dissociating state. The Aro mutants are defective in forming the slowdissociating complex with 20 nt DNA and are also not able to efficiently destabilize
secondary DNA structures. In DNA repair, intermediates contain short ssDNA regions
and partially duplexed DNA structures. We conclude that defects in complex stability and
destabilizing partially duplexed structures are the cause of the loss of Aro mutant activity
in DNA repair and that the slow-dissociating state of RPA is needed for correct
processing of these ssDNA intermediates.
Materials and methods
Protein purification
Biotinylated RPA3 was made by synthesizing a synthetic coding sequence that
contained a XbaI site, an N-terminal BirA recognition sequence
(BAP:GLNDIFEAQKIEWHW) (141), a six histidine His-Tag, and the coding sequence
for RPA3 with codon usage optimized for expression in E. coli followed by a BamHI site
(Genscript). This sequence was then used to replace the existing RPA3 gene in p11dtRPA containing wild-type RPA (142) using XbaI and BamHI. The new plasmid, p11dtRPA•RPA3biotin directs the expression of RPA1, RPA2 and biotin-RPA3 as a synthetic
operon in E. coli. To make biotinylated Aro mutants, the AroA, AroB and Aro2 coding
sequence from pRSF–AroA, –AroB and –Aro2 were each excised with SfiI and AvrII
sites and used to replace the wild-type RPA1 subunit in p11d-tRPA•biotinRPA3 (cut at
SfiI and NheI sites). Biotinylated proteins were purified as previously described for nonbiotinylated RPA (143), with the exception that 100 µM biotin was added to the LB
media concomitant with the induction of 0.3 mM IPTG.
28
DNA oligonucleotides
dT35, dT25, dT20 , dT15 ssDNA (IDT) used in the single molecule experiment
all have Cy3 fluorophore at their 5’ ends. RFL (replication fork like) DNA was annealed
from four different oligonucleotides and contains both Cy3 and Cy5 dyes. Lagging
strand: Oligo-G
5’CGTACTGCAATCTTGAACCG(T)20/Cy3/GGAATTAAGCTCTAAGCCATCC 3’,
Oligo-H 5’ /Cy5/CGGTTCAAGATTGCAGTACG 3’; Leading strand: Oligo-I 5’
GCGTGATAGCATCCATGAGC 3’, Oligo-J 5’
GGATGGCTTAGAGCTTAATTCCGCTCATGGATGCTATCACGC 3’.
GAP DNA was a modified RFL made by annealing the lagging strand’s two
oligonucleotides with Oligo-JB 5’ GGATGGCTTAGAGCTTAATTCC 3’.
20 nt bubble DNA was annealed from: Oligo-BB 5’Cy3
CCCTAGATACCAGTAAGCCTAAGGCCGGATCTCGGGCCATCCATGTACGC 3’,
Oligo-BT
5’GCGTACATGGATGGCTTAGAGCTTAATTCCGAATCTACTGGTATCTAGGG/C
y3/ 3’
For all of the annealed DNA structures, underlines indicate complementary
sequences, which are annealed in the final product. Annealing is carried out by mixing 2
µM DNAs at an annealing buffer containing 30 mM Tris-HCl (pH 7.5), 150 mM NaCl,
and 0.5 M EDTA, and was denatured at 95 °C for five minutes and allows cooling down
to the room temperature for 2 hours. The annealed products were stored in -4 °C.
Reaction conditions for the single-molecule assays
Biotinylated RPA and Aro mutants were immobilized on a quartz surface
(Finkenbeiner) coated with polyethylene glycol (PEG) to eliminate non-specific binding.
The immobilization was mediated by neutravidin-biotin interaction between biotinylated
proteins, neutravidin (Pierce), biotinylated polymer (Laysan Bio, MW5000; mPEG-SVA
29
and biotin-PGE-SVA). Details for preparing the slides and chambers for single molecule
experiment were as previously described (144). All single molecule experiments were
performed in binding buffer: 50 mM Tris-HCl (pH 7.5), 100 mM NaCl, 5 mM MgCl2,
100 ng/µl BSA. Imaging was done in imaging buffer (binding buffer supplemented with
1mg/ml of Trolox (6-hydroxy-2, 5,7,8-tetramethylchroman-2-carboxylic acid; SigmaAldrich), 1mg/ml of glucose oxidase (Sigma-Aldrich), 0.4% (w/v) D-glucose (SigmaAldrich) and 0.04 mg/ml of catalase (Calbiochem)). In each experiment, 100 µl of 0.2
mg/ml of Neutravidin dissolved in PBS was flowed into the assembled chamber,
incubated for five minutes, followed by 100 µL of 50 pM of Biotinylated protein in
binding buffer. After another five minute incubation, 100 µL of 100 pM of Cy3-and
Cy5-labeled DNA indicated substrate was flowed into the chamber diluted in imaging
buffer with binding buffer at last. In some experiments as a final control, the chamber
was washed extensively with binding buffer and Cy3-labeled dT35 added to detect all
tethered RPA. The concentrations were optimized to detect 500-1000 individual
molecules per experiment (50 pM of biotinylated RPA and 100 pM Cy3-labeled DNA).
Single-molecule smTIRF
Single molecule experiments were carried out with a prism-type TIRF microscope
as previously described (145). Cy3-labeled substrates were excited by a DPSS laser (532
nm, 75 mW, Coherent), whereas a diode laser (641 nm, 100 mW, Coherent) was used to
excite Cy5 labeled substrates (146). The florescence signals coming from Cy3 and Cy5
dyes were collected using a water immersion objective 60x (Olympus), separated by a
630-nM dichroic mirror, passed through a 550-nm long-pass filter to block out laser
scattering and recorded with a EMCCD camera (Andor) (time resolution of 100 ms).
smTIRF Data analysis
The single molecule trajectories were extracted from the recorded video files
using in-house IDL software. Individual trajectories were visually inspected and picked
30
using MATLAB. The picked individual trajectories were analyzed by QUB for acquiring
“on times” and “off times”. The measured “on times” and “off times” from all RPA
molecules were combined and binned to plot as a histogram and fit to single-exponential
or double exponential equation in GraphPad Prism 6.0 software to obtain respective rate
constants. The extracted “off times” were binned with the bin size of 3 sec and “on
times” were binned with the bin size of 0.5 sec. To decide the optimal bin size, we
initially utilized the web application for bin-width optimization (Ver.2.0) from
Toyoizumi lab. After binning several data sets from RPA binding to dT35, we evaluated
the quality of the fit, how the bin size affected the binding parameters and the amount of
“noise” in the histograms. Based on these criteria, we were able to determine the optimal
bin size for “on times” and “off times” and applied them for all our data to keep the
fitting of the histograms consistent. The dissociation rate was calculated from fitting the
“on times” distribution to one or two phase exponential decay, yielding the apparent
dissociation rate constants koff (s-1). The apparent rate association constants kon (M -1 s-1),
were calculated as fitting the “off times” to one or two phase exponential decay to obtain
the number of events per sec (s-1) and divided by the DNA concentration. Ka=kon/koff (M1
)
Electrophoretic mobility assay and helix destabilizing assay
An electrophoretic mobility assay was used to determine binding affinity of RPA
and mutants to Replication fork like (RFL) DNA structure, GAP and Bubble DNA. 6 nM
DNA was incubated with increasing amounts of indicated form of RPA in a total volume
of 15 µL binding buffer (50 mM Tris-HCl (pH 7.5), 100 mM NaCl, 5 mM MgCl2, 100
ng/µl BSA) for 20 minutes at room temperature. DNA-protein complexes were separated
on a non-denaturing 3.5% acrylamide gel (1X TAE, 3.5% Acrylamide, TAMED, 10%
APS). The running buffer was 1X TAE (40 mM Tris, 20 mM Acetic acid, 1 mM EDTA).
The Cy3 signals from the free and bound DNA were visualized by Chemidoc MP
31
imaging System (Bio-Rad). In helix destabilization assays, at the end of the 20 minute
incubation, 2% (w/v) SDS, 1mg/ml proteinase K (Qiagen) was added to denature protein
and a large excess of competitor DNA (5µM) that was complementary to the non-labeled
strand of DNA was added to prevent re-annealing. Reactions were separated on a 15%
acrylamide gel to distinguish intact DNA structures from melted ssDNA.
Results
ssDNA interaction with surface-tethered RPA
Mutation of the conserved aromatic residues in the DNA-binding sites of DBD-A
and DBD-B causes defects in DNA repair but not in replication (120). This suggests that
replication and repair require different RPA-DNA interactions. While it was known that
these aromatic residue mutants alter interactions with short ssDNA regions (120), it is not
known how these mutations affect kinetics of binding or the binding to partially-duplexed
DNA intermediates. Therefore, we carried out single molecule analysis in which
biotinylated RPA was immobilized on a slide surface and allowed to interact with freely
diffusing fluorescent-labeled DNA substrates (147). This approach allows direct
observation and analysis of binding events in real time (148). The major advantage of
studying the binding at the level of individual molecules lies in the direct measurement of
distributions of molecular events, rather than their ensemble averages (148). By
constructing histograms of individual molecules, aberrant subpopulations can be
identified and characterized (148). Also, the recording of single-molecule trajectories
allows us to observe rare and short-lived intermediates (148). Kinetics parameters can be
obtained under the equilibrium conditions. To make biotinylated RPA and Aro mutants,
RPA3 contained an N-terminal recognition sequence for BirA biotin ligase was cloned
into the construct for expressing RPA in E. coli constructs (Figure 2.1 A) (141,146). The
resulting forms of RPA were expressed and purified (Figure 2.1 B). The purified
32
biotinylated forms of RPA were active and have apparent association constants similar to
the unbiotinylated forms (Figure 2.1 C).
Purified biotinylated RPA was immobilized on neutravidin-coated quartz slides
that were blocked with polyethylene glycol (PEG) to prevent non-specific protein binding
(144). The concentration of biotinylated-RPA and Aro mutants was optimized at 50 pM
to ensure separation between immobilized molecules on the slide. At this concentration,
500-1000 molecules were observed on slides simultaneously when fluorescently labeled
DNA was added.
Surface-tethered biotinylated RPA or Aro mutants were then incubated with Cy5
and/or Cy3 labeled DNA and the slide illuminated with the 532 nM green laser to achieve
total internal reflection (TIR). Dye molecules present within the evanescent field
generated by the TIR (generally within 100-150 nM of the slide surface) are directly
excited (Figure 2.2 A), so the fluorescent signal is only observed when a DNA molecule
is bound to the immobilized RPA (Figure 2.2 A). 532 nM light directly excites Cy3
emission and in DNA molecules with both Cy3 and Cy5, a Cy5 signal is observed due to
fluorescence resonance energy transfer (FRET) between Cy3 and Cy5 dyes. Unbound
DNA outside of the evanescent field is not excited and not detected. In the absence of
biotinylated RPA protein, there are no binding events with just fluorescently labeled
DNA added. The camera time resolution was set to 100 mS to exclude short binding
events that are likely result from non-specific DNA binding.
The advantage of this experimental approach is that a single molecule of RPA is
able to bind to multiple ssDNA molecules over time, yielding a fluorescent trajectory
(example shown in Figure 2.2 B). The fluorescent signal at the location of a molecule of
RPA is characterized by a series of fluorescent pulses in which the duration of each pulse
corresponds to an “on time” of a binding event (i.e. the amount of time that a
fluorescently-labeled DNA molecule is bound to the RPA). The times between pulses,
where there is minimal fluorescent signal, are defined as “off times” and correspond to
33
protein alone (Figure 2.2 B). We consistently observed individual surface-tethered RPA
molecules undergoing multiple binding events.
DNA-binding of surface-tethered RPA
Initial experiments monitored the interactions of surface-tethered, wild-type RPA
and Cy3-labeled dT35. The acquired “on times” and “off times” of all trajectories were
combined and plotted as histograms in which the numbers of events are plotted against
time (Figure 2.2 C). This distribution can fit to one or more exponential decay terms,
enabling us to retrieve the rate constants and fraction of each term. In order to determine
the best fit for the data, fits to one-phase decay or two-phase decay were compared by
sum-of-squares F test (Prism). This method selects the simpler model unless the P-value
for the more complex model is less than 0.05. Also, if one fit was ambiguous (e.g. fitting
does not return a unique set of parameters), the other fit was chosen without formal
comparison. For wild-type RPA binding to dT35, the “off times” fit best to a one phase
exponential equation with a von= 0.032 ± 0.001 s-1. As the von is obtained from 100 pM
dT35 DNA, the kon = von / 100 pM = 3.20 ± 0.14*108 (M-1s-1). Because kon is dependent
on the DNA concentration, we did a titration of DNA concentrations from 100 pM to 300
pM and plotted von (s-1) for each DNA concentration (Figure 2.2 D). The resulting von
values were linearly dependent on DNA concentration (Figure 2.2 D) and kon (the slope)
was determined to be 3.1× 108 M-1s-1. Based on the linear dependence of von, we used 100
pM DNA for all subsequent studies.
The “on times” of wild-type RPA fit best to a two-phase exponential decay, with
koff-fast= 0.15 ± 0.05 s-1 and koff-slow= 0.02 ± 0.01 s-1 and the fast component comprising
~82% of the total events (Table 2.1). The binding of wild-type RPA to dT35 is best
described by two equilibrium constants: Kafast= 1.47 ± 0.27 × 109 M-1 and Kaslow=1.66 ±
0.59 × 1010 M-1, which were calculated by koff-fast and koff-slow divided by kon. Kaslow is
similar to the equilibrium binding constants determined from GMSA (Figure 2.1 C) and
34
in previous analysis of RPA (31,51). We conclude that immobilization of RPA did not
affect the ability of the protein to bind to ssDNA.
The observation that RPA dissociation has a fast and a slow phase is novel. These
two phases have not been identified in previous DNA binding analysis (54) These kinetic
parameters could not have been determined in indirect assays such as GMSA. This
analysis suggests that there are at least two states in RPA-DNA complexes. These
studies cannot identify what these two states are; though, they could reflect different
domains interacting with the DNA or different conformations.
Aro mutants have reduced binding to short ssDNA
We then determined the binding parameters for the three aromatic residue mutants
with repair defects: RPA-AroA, RPA-AroB and RPA-Aro2. The DNA binding
parameters obtained for the Aro mutants with dT35 RPA were similar to those for wildtype RPA; the Aro mutants had on-rates and off-rates for dT35 similar to that of wildtype RPA (Table 1). As with wild-type RPA, dissociation fit better to a two-phase
exponential with the faster off-rate predominating for all three mutants (>78% fast rate,
Table 2.1).
We next examined interactions of the different forms of RPA with
oligonucleotides of shorter lengths: dT25, dT20 and dT15. Wild-type RPA, AroA, AroB
and Aro2 bound to dT25 and dT20 with an affinity similar to that observed with dT35
(Figure 2.3 A & 2.3 B). However, with dT20, two-phase dissociation was only observed
with wild-type RPA. The dissociation of AroA, AroB and Aro2 fit best to a single offrate that was similar to the fast rate observed with dT35 and dT25 (Table 2.1). This
suggests that in spite of having a high affinity for dT20, Aro mutants interact differently
with the DNA than wild-type. With dT15, wild-type RPA bound with an affinity similar
to dT35 while no binding events were observed with any of the Aro mutants.
35
These experiments showed that RPA bound with high affinity to ssDNA as short
as 15 nt in length while the Aro mutants bound with high affinity to dT20 but not to
dT15. When complexes formed, the forms of RPA bound with similar affinity. The onrates (1 × 108 M-1s-1 to 4 × 108 M-1s-1) indicated that association of all forms of RPA is
close to the diffusion limit (Table 2.1). Dissociation of Aro mutant complexes with dT25
and dT35 was best described by a two phase exponential (Table 2.1). This suggests that
like wild-type RPA, the aromatic residue mutants have multiple states when they form
complexes with >25 nt oligonucleotides. Strikingly, the Aro mutants did not form two
states with dT20 and were not able to form stable complexes with dT15 at all (Table 2.1).
This confirms previous analysis that these mutants have altered interactions with short
oligonucleotides (120).
Aro mutants are defective in forming “long-lived”
complexes
Previously, these Aro mutants were shown by GMSA to have an apparent affinity
for dT30 that was one tenth of that wild-type RPA ((31,120); Figure 2.1 C). However, in
our TIRFM studies that directly monitor individual binding events, all three Aro mutants
have binding parameters that are the same as wild-type RPA. This suggests that these
two assays are measuring different properties. In particular in the GMSA, RPA-DNA
complexes must remain stable during electrophoretic separation to be measured. So a
lower apparent affinity in GMSA suggests that the aromatic residue mutations are
affecting the stability of the RPA-DNA complexes during electrophoresis. To explore
this difference, we quantitated the fraction of “long-lived” RPA-DNA complexes for each
form of RPA as a function of DNA length (Figure 2.4 A). The mean dwell-time for
RPA•dT35 complexes was ~40 sec so we determined the fraction of each population of
RPA-DNA complexes with dwell-times longer than 40 seconds. For wild-type RPA
binding to dT35, ~26% of the binding events were longer that 40 seconds. This fraction
36
decreased with shorter DNA lengths until only one percent of the wild-type RPA•dT15
complexes had dwell time of 40 seconds or longer (Figure 2.4 B). We conclude that
wild-type RPA forms less stable complexes with shorter ssDNA.
The Aro mutants had a stronger length-dependence of binding. With dT35, all
three Aro mutants had approximately 25% of binding events with dwell times longer than
40 sec. With dT20, wild-type RPA had still had 14% long events while AroA had 2%
and AroB and Aro2 did not have any events longer than 40 sec. (Figure 2.4 B) (Table
2.3). We conclude that the Aro mutants are defective in forming “long-lived” complexes
when binding to intermediate and short length ssDNA. The loss of “long-lived”
complexes correlated with the disappearance of the slow-off rate phase for the Aro
mutants. This suggests that the slow-off phase represents a more stable complex that
does not form when the Aro mutants bind to dT20. Even though the long binding events
are in a small portion of the total binding events, we speculated that the ability for RPA to
form long binding events might be important for RPA function in DNA repair.
Aro mutant binding to partially duplex DNA structures
Intermediates that form during DNA replication and repair have ssDNA regions
of different lengths and are either at the end of or adjacent to duplex DNA. To determine
whether the structures adjacent to a single stranded region affect Aro mutant binding, we
next tested binding to partially duplexed DNA structures. DNA structures resembling a
stalled replication fork (RFL-replication fork like), a single stranded gap (GAP) and
single stranded bubble (Bubble) were examined. Each of these structures was made by
annealing appropriate synthetic oligonucleotides and had a 20-nucleotide ssDNA region
(Figure 2.5 A, B and C). The oligonucleotides were labeled with Cy3 or Cy5 as indicated
so that annealing and subsequent melting of the partially duplexed structures could be
directly monitored (Figure 2.5 A, B and C).
37
TIRFM analysis showed that wild-type and the Aro mutants bound the RFL and
GAP DNAs with an affinity similar to that of dT20 (Figure 2.6) and had similar kinetic
parameters (Table 2.2 and Table 2.4). Both Cy3 and Cy5 channels were monitored for all
binding events. This showed that under these conditions RPA binding generally does not
cause melting of the partial duplex structures (data not shown and see also below). Rare
binding events in which only a single labeled oligonucleotide was present were not
analyzed. With both RFL and GAP DNA, all forms of RPA dissociated with two-phase
kinetics (Table 2.2). For the Aro mutants, this is different than binding to dT20, which
was best described by a single off-rate. We speculate that this difference is the result of
“breathing” of the duplex adjacent to the ssDNA region, which results in these templates
having a slightly longer effective ssDNA region for RPA binding.
To confirm these findings and explore if there were differences in the DNA
complexes formed, we also examined binding on native gels (GMSA). Wild-type RPA
and Aro mutants bound RFL and GAP DNAs with similar affinities (Figure 2.7 B and
Figure 2.8 B). RPA•DNA complexes formed with similar concentrations of each form of
RPA (Figure 2.7 B and 2.8 B) leading to similar apparent dissociation constants (Table
2.2). However, a slower mobility complex was observed with RFL DNA at high
concentrations of wild-type RPA (Figure 2.7 B). This complex was not observed with
GAP DNA (Figure 2.8 B). A very small amount of this complex was also observed at the
highest concentrations of AroA and Aro2 but it was never observed with AroB. This
suggests that wild-type RPA can form additional complexes with the replication fork-like
structure, which are not formed efficiently (or at all) with the Aro mutants.
To determine whether RPA and Aro mutants binding to RFL and GAP caused
melting of the duplex regions in the gel assays, we carried out helix destabilizing assays
in parallel. In these assays, RFL or GAP DNA was incubated with protein as in GMSA
and then complexes were disrupted with SDS, proteinase K in the presence to excess
unlabeled ssDNA (to prevent the labeled oligonucleotide from reforming into duplex
38
structures). The DNA was then analyzed on 15% polyacrylamide gels to determine
whether any melting had occurred. No melting was observed for RFL and GAP DNAs
with either RPA or the Aro mutants (Figure 2.7 B and 2.8 B). These experiments suggest
that melting was not occurring (and is not required for) RPA binding to the 20 nt gap in
either of these structures.
RPA and Aro mutants binding to Bubble DNA
We also examined binding to duplex DNA containing a 20 nt single stranded
region surrounded by duplex DNA (a bubble; figure 2.9 A). In GMSA assays, wild-type
RPA was able to bind the Bubble DNA at high concentrations. Complexes were
observed at 5- and 10-fold molar excess of RPA (Figure 2.9 B). In contrast, there was
minimal binding by the Aro mutants as the same concentrations (Figure 2.9 B). (Note the
minimal decrease in the amount of free DNA with Aro mutants.) When similar reactions
were analyzed for helix destabilization, wild-type RPA caused melting of the Bubble
DNA at the same concentrations as complex formation was observed (Figure 2.9 B).
This suggests that the stable RPA-bubble complex formation is the result of melting the
Bubble DNA or occurs concurrently with melting. Binding to Bubble DNA also required
higher concentrations than was required for binding to either RFL or GAP DNA
(compare Figures 2.7 B and 2.8 B) and only occurred when the stoichiometry of RPA to
DNA was ≤1:5. This suggests that multiple RPA molecules are needed to bind and melt
Bubble DNA. In contrast with wild-type, the Aro mutants were unable to melt the
Bubble DNA. We conclude that the Aro mutants are defective in forming a stable
complexes/melting Bubble DNA.
To further examine interactions with Bubble DNA, we assessed binding in
smTIRFM. Strikingly, no binding events were detected with either wild-type or the Aro
mutants. In these smTIRFM experiments, the density of tethered-RPA on the slide
surface is low enough that each binding event represents DNA molecules binding to
39
individual molecules of RPA. (Note that if multiple RPA molecules were located at a site
on a slide, they would have been detected in the oligonucleotide binding studies as spots
with double fluorescence intensity (2 DNA molecules) binding events and these were not
observed.) These data confirm that binding of multiple RPA molecules are needed for
binding to and melting of Bubble DNA.
The binding behavior of the Aro mutants was very different with the Bubble
substrate relative to the GAP DNA; even though both contain a 20 nt single strand region.
To explore this difference we also examined melting of DNAs containing either 5’ or 3’
flaps (Figure 2.10 A). These DNAs are equivalent to the bubble substrate with a nick at
either the 3’ or 5’ end of the bubble: a 20 nt single stranded region adjacent to duplex
DNA on one side and a 20 nt flap on the other. No melting was observed with either flap
substrate (Figure 2.10 B). We conclude that (i) the topological constraints present in the
20 nt bubble prevent stable binding unless the adjacent duplex regions are melted, (ii)
that this melting requires binding of multiple molecules of RPA and (iii) that the Aro
mutants are defective in this binding/melting activity.
Discussion
RPA plays essential roles in DNA synthesis and repair pathways. It is known that
RPA binds ssDNA with high affinity and interacts with protein partners to function in
different pathways. However, the unique functions of RPA remain incompletely
understood. RPA is composed of multiple DNA-binding domains connected by flexible
linkers. In addition, a variety of ssDNA intermediates are found in cells. These
intermediates have different lengths and locations and need to be processed by different
pathways. Therefore, detailed understanding of the molecular basis of RPA-DNA
interactions is needed to explain how RPA can function in different pathways with the
various lengths of ssDNA and partial duplex DNA structures presented in cells. The goal
of these studies was to directly monitor the interactions and kinetics of RPA and a set of
40
repair-defective Aro mutants binding to different forms of single stranded and partially
duplex DNAs. We applied single molecule total internal reflection fluorescence
microscopy to study surface-tethered RPA and Aro mutants binding to freely diffusing
fluorescently labeled DNA. These studies visualized binding events based on fluctuation
of a fluorescent signal at individual locations inside the smTIRFM chamber. This
fluctuation is caused by DNA substrates being retained in the evanescent field as they
associate and dissociate from individual tethered RPA molecules. In these experiments,
individual molecules of RPA undergo multiple binding cycles. By analyzing large
numbers of individual binding events, we observed that there is heterogeneity in binding
of both wild-type and the Aro mutant forms of RPA. This manifested as two phase
dissociation of the RPA•DNA complexes.
Our analysis of single molecule binding data showed that Aro mutants have
reduced binding to the short length ssDNA and also are missing a more stable bound state
that was observed when either wild-type or Aro mutants bound longer ssDNA. The Aro
mutants function in DNA replication but not in DNA repair. In DNA replication the
ssDNA intermediates are generally transient and have lengths usually around 100-200 nt.
In contrast the ssDNA intermediates nucleotide excision repair are much shorter (<30 nt)
and topologically constrained. Our data suggest that the reduced interactions with and
inability of the Aro mutants to form stable complexes with short ssDNA is likely to be
the cause of the defect in NER.
RPA is a modular protein with multiple, independent DNA-binding domains. The
flexible structure of RPA has been suggested to be responsible for multiple modes of
DNA binding that differ in length of DNA covered and number of domains engaged
(32,53,140,149,150). These studies indicate that formation of stable RPA•DNA
complexes requires not only the high affinity binding domains DBD-A and DBD-B, but
also DBD-C and DBD-D. We showed that the Aro mutants have similar binding affinity
as RPA to ssDNA 20 nt or longer. This result indicates that by having multiple DBDs,
41
RPA binding to ssDNA is resistant to mutations that partially disrupt the binding of a
single domain; i.e. that the reduced binding of mutated single domain can be partially
compensated by binding of the other three domains. The fact that the affinity of RPA for
oligonucleotides from 20 to >30 nt is similar indicates that 20 nt is sufficient to
accommodate binding of four DBDs. These results correlate with the previous finding
that that RPA binds 5’- and 3’- protruding ssDNA with different affinity if the length of
the arm is 19 nt or shorter but has similar affinities when length of the arm is 23 nt or
longer (47). RPA binds with a specific polarity and the shorter ssDNA arms restricted
different DBDs from binding depending on the orientation of the protruding arm.
We also analyzed the fraction of complexes with long dwell-times and found that
the occurrence of long binding events decreased with shorter ssDNA lengths. With dT35,
there is no difference in the fraction of long dwell-time complexes between RPA and Aro
mutants. However, Aro mutants show the decrease in the long dwell-time complexes
with intermediate length ssDNA (dT25 and dT20). With wild-type RPA there was a
large decrease in the fraction of long dwell-time complexes with dT15 (The Aro mutants
did not form stable complexes with dT15). These data are also consistent with the model
that multiple domain interactions are required for the most stable complexes. dT15 is too
short to allow the domain interactions needed for formation of stable long dwell-time
complexes. This means that with dT15, RPA-DNA interactions are primarily mediated
by the high affinity binding domains A and B. This makes binding to dT15 very
sensitive to mutations in DBD A or B. With intermediate lengths ssDNA, the
contribution of domains outside of the high affinity domain is partial. The other domains
contribute which leads to high affinity binding but the more stable complex does not
seem to form.
The formation of long dwell-time complexes seems to be important for RPA
melting of secondary DNA structure (151). Indeed, the Aro mutants are unable to unwind
a 20 nt Bubble DNA structure. However, Aro mutants show high binding affinity to 20 nt
42
RFL, GAP, and flap containing structures. This indicates that binding to topologically
constrained DNA structures like Bubble DNA requires either the full activity of DBD-A
and DBD-B or formation of the more stable, slow-disassociating complex or both. (It
seems likely that highly stable binding is required for RPA melting.) It’s been suggested
that when one RPA binds to ssDNA with low affinity, cooperative binding of an
additional RPA can significantly strengthen the RPA-DNA interaction (152). In this case,
when RPA binds to the bubble DNA, RPA first needs to bind to the ssDNA region of
bubble (which because of topological constraints “acts” like it is shorter than 20 nt), and
then destabilize the helix to make a larger region of ssDNA to form the stable complex.
We hypothesize that RPA binding causes some unwinding of the duplex regions which
allows/requires binding of a second RPA molecule and eventually complete melting.
This model explains why we did not observe unwinding for RFL, GAP or flap containing
molecules even in the presence of a 10-fold molar excess of RPA. Because these forms
of DNA are not topologically constrained, binding can occur without, or with minimal,
melting of adjacent duplex DNA.
Recent studies suggested that RPA binding is very dynamic (54,122). The finding
that dissociation of RPA fits best to two-phase exponential decay indicates that the RPA
binding to ssDNA is not simply sequential engagement of DBDs ending in a single 30-nt
complex. There are at least two populations of “states”, with fast and slow off rates. Our
kinetic studies do not indicate the structures or domains required for the different states.
However, to achieve long binding events, it’s likely that all DBDs need to contact with
ssDNA. Previous studies showed that the high affinity-binding core (DBD-A and –B)
can bind to ssDNA with high affinity (32,33). Also the RPA trimerization core (DBD-C,
-D, and –E) is capable of binding to partial duplex with a 5’ssDNA overhang of either 10
or 30 nt (27,138). A progressive compaction of RPA upon binding to different length
DNA has been observed using SAXs and cryo-EM (52,153). Also a recent analysis
suggested that human RPA can interact with DNA with a binding site of either 22 or 30
43
nt at different ionic strengths (54) which is consistent with there being either 3 or 4 DBDs
interacting with DNA. Together these studies suggest that RPA forms different
complexes with ssDNA and that the DBDs can interact differentially in different
complexes. It is clear that with short DNAs, DBD-A and DBD-B binding is essential for
the formation of stable complexes but that with longer DNA, partial defects in DBD-A
and DBD-B are tolerated. We hypothesize that initially one or more DBDs interact with
DNA after a diffusion dependent collision. More DBDs associate to form a stable
complex in which 3 or 4 DBD are generally associating with the DNA. There is evidence
for microscopic dissociation of individual domains but overall it appears that there are
multiple interactions in the stable complex. Our data indicates that there are at least two
kinetic states in these complexes. These could differ in the number of DBDs associated
with the DNA or by some conformational change in the complex as modeled in Figure
2.11. However our data is unable to distinguish the molecular basis of these states.
The interaction of RPA and DNA are complex. By studying the four-conserved
aromatic resides in high affinity domain of RPA, we revealed that binding to short
ssDNA is not required for RPA to function in replication but is essential for repair
processes. We also identified that RPA•DNA complexes exist in at least two kinetic
states. Future studies are needed to determine how the domains of RPA contribute to
these two states and how these RPA•DNA complexes contribute to processing of
different single-stranded DNA intermediates.
44
Figure 2.1. Biotin is covalently linked to the RPA3 subunit of RPA to surface tether RPA
in smTIRF.
(A) The recognition sequence for the E.Coli BirA biotin ligase (BAP) was added
to RPA N terminus of RPA3 subunit in construct that is known for expression of RPA.
When the construct is transformed in DE3 cells, the E.coli BirA biotin ligase covalently
links biotin to the lysine within the BAP sequence of RPA3. Schematic representation
shows the spatial localization of Biotin on RPA3 subunit relatively to the RPA complex.
(B) Silver staining stained PAGE confirming expression and purity of biotinylated RPA
and Aro mutants. (C) Gel mobility shift assay (GMSA) confirmed the binding activity of
biotinylated RPA and Aro mutants. Increased amount of the indicated proteins (0-1000
fmol) were incubated with 2 fmol of radiolabeled dT35. The association constants from
three separate experiments were determined and the average and standard deviation (error
bars) is shown.
45
A
C
B
46
Figure 2.2. Surface-tethered RPA shows binding activity.
(A) Schematic representation of TIRFM-based assay for analysis of DNA binding
by the individual RPA molecule. RPA is immobilized on the surface of the microscope
flow cell and an evanescent filed was generated on cell surface by TIR when illuminated
with a 530 nM laser. The DNA substrates labeled with Cy3 is only visible unless found
within the evanescent field because of its association with the surface-tethered RPA.
Dissociation of DNA will lead to loss of signal. (B) A representative trajectory for a
single RPA binding to ssDNA. Binding activity of an individual RPA was monitored
continuously for 6000 s in the presence of Cy3-labeled dT35. Each spike in the
fluorescence intensity of Cy3 (green) corresponds to binding and dissociation of a new
DNA substrate. The length of binding event is “on time”, and dissociation time between
two binding events is counted as “off time”. In each experiment, there are 600-1000
trajectories originated from individual surface-tethered RPA molecules similar to the one
depicted here. (C) kon and koff are determined for RPA binding to dT35 using smTIRF.
The “off times” and “on times” from individual RPA binding to dT35 (100 pM) are
combined. Distributions of “off times” (upper panel) and “on times”(lower panel) are fit
to one or double exponential decay to obtain vondecay and koff-fast, koff-slow as shown. (D) The
vondecay is acquired for RPA binding to dT35 at concentration 0.1 nM, 0.2 nM and 0.3 nM.
The DNA concentration and vondecay shows a linear relationship and kon (s-1M-1) is
the slope.
47
A
B
C
D
48
Figure 2.3.Mutation of aromatic residues affect DNA binding to short ssDNA.
(A) Binding affinity of RPA and Aro mutants was measured with smTIRF to the
indicated DNA substrates. Association constant from two phase exponential decay, Kafast
(left) and Kaslow (right), is determined based on the kon and koff-fast and koff-slow . Error bars
come from the standard deviation of three independent experiments. No koff-fast and koffslow is
available for AroA and AroB with dT20 (because they did not fit to two phase), so
Kafast and Kaslow is labeled ** for AroA and AroB with dT20. No complex was detected
(ND) for AroA, AroB and Aro2 with dT15. (B) The association constant Ka from one
phase exponential decay is determined based on the kon and koff from the same experiment
as above. Error bars come from the standard deviation of three independent experiments.
No complex was detected (ND) for AroA, AroB and Aro2 with dT15.
49
A
B
50
Figure 2.4.The fraction of long-dwell complexes is dependent on length of ssDNA.
(A) The “dwell time” distribution for RPA binding to dT35, dT25, dT20 and
dT15 were shown. 40 sec is the cut off point, presented by the red line. (B) Quantification
of the fraction of long dwell-time that is longer than 40 sec for RPA, AroA, AroB and
Aro2 binding to the indicated length of ssDNA is shown. The errors for the fraction are
standard errors from average of three independent experiments. No long-dwell complex
(>40 sec) was detected (ND) for AroB and Aro2 with dT20 and dT15, and AroA with
dT15.
51
A
B
52
Figure 2.5. Making partial duplex DNA structures.
The schematic representation of structure of 20 nt bubble, RFL and GAP (above).
20 nt bubble (A) is annealed from two oligos. Annealed products are examined using
15% poly acrylamide gel. RFL (B) is annealed from four different oligos (G, H, I, J).
GAP (C) is annealed from three different oligos (G, H, JB). Adding trap DNA and
boiling could denature the DNA structure, yielded ssDNA labeled with Cy3 or Cy5 (left
most band). Annealing different combination of indicated oligos yielded different band
locations on gel. 20 nt bubble is Cy3 labeled and is checked by Cy3 channel of camera.
For annealing products of RFL and GAP, the same gel is looked under both Cy3 and Cy5
channel.
53
A
C
B
54
Figure 2.6. RPA and Aro mutant show high affinity toward RFL and GAP in smTIRF.
The association constant Ka from one phase exponential decay is determined
based on the kon and koff. Error bars come from the standard deviation of three
independent experiments.
55
Figure 2.7. RPA and Aro mutants show high affinity toward RFL with no helix
destabilization.
(A) Schematic representation of RFL and position of Cy3 and Cy5 were shown.
(B) RFL-binding and (C) helix destabilization activities of RPA and Aro mutants. The
indicated amount of RPA and Aro mutants were incubated with 6 nM of fluorophorelabeled RFL at room temperature for 25 minutes. The reactions were terminated and
separated by electrophoresis as described under materials and methods for RFL binding
(B) and helix destabilization (C). (B) The positions of the DNA-protein complex and free
DNA were indicated. (C) The position of RFL and ssDNA were indicated. Boiled
control produces Cy3 and Cy5-labeled ssDNA to show the position of melted ssDNA.
56
A
B
C
57
Figure 2.8. RPA and Aro mutants bind GAP DNA with high affinity with no helix
destabilization.
(A) Schematic representation of GAP and position of Cy3 and Cy5 were shown.
(B) GAP-binding and (C) helix destabilization activities of RPA and Aro mutants. The
indicated amount of RPA and Aro mutants were incubated with 6 nM of fluorophorelabeled GAP at room temperature for 25 minutes. The reactions were terminated and
separated by electrophoresis as described under materials and methods for GAP binding
(B) and helix destabilization (C). (B) The positions of the DNA-protein complex and free
DNA were indicated. (C) The position of GAP and ssDNA were indicated. Boiled
control produces Cy3 and Cy5-labeled ssDNA to show the position of melted ssDNA.
58
A
B
C
59
Figure 2.9. Aro mutants fail to stably associate with Bubble DNA and show defective in
melting activity.
(A) Schematic representation of 20 nt bubble DNA and position of Cy3 was
shown. Bubble binding (B) and helix destabilization (C) activities of RPA and Aro
mutants. The indicated amount of RPA and Aro mutants were incubated with 6 nM of
fluorophore-labeled bubble at room temperature for 25 minutes. The reactions were
terminated and separated by electrophoresis as described under materials and methods for
bubble binding (B) and helix destabilization (C). (B) The positions of the DNA-protein
complex and free DNA were indicated. (C) The position of 20 nt bubble and ssDNA were
indicated. Boiled control produces Cy3-labeled ssDNA to indicate the position of melted
ssDNA.
60
A
B
C
61
Figure 2.10. RPA and Aro mutants do not melt DNAs containing 5’ or 3’ flaps.
(A) schematic representation of DNA containing 5’ flap (left). Helix
destabilization activities of RPA and Aro mutants (right). The indicated amount of RPA
and Aro mutants were incubated with 6 nM of fluorophore-labeled 5’ flap at room
temperature for 25 minutes. The reactions were terminated and separated by
electrophoresis as described under materials and methods for helix destabilization (right).
(right) The position of 5’ flap and ssDNA were indicated. Boiled control produces Cy3labeled ssDNA to indicate the position of melted ssDNA. (B) Schematic representation of
DNA containing 3’ flap (left). Helix destabilization activities of RPA and Aro mutants
(right). The indicated amount of RPA and Aro mutants were incubated with 6 nM of
fluorophore-labeled 3’ flap at room temperature for 25 minutes. The reactions were
terminated and separated by electrophoresis as described under materials and methods for
helix destabilization (right). (right) The position of 3’ flap and ssDNA were indicated.
Boiled control produces Cy3-labeled ssDNA to indicate the position of melted ssDNA.
62
A
B
63
Figure 2.11. Model of RPA multi-step dynamic binding.
64
Table 2.1. Different length of ssDNA binding by RPA and Aro mutants.
kon shows as one phase. koff shows as two phase: koff-fast and koff-slow. koff for one
phase was also shown. ND=Not Detected. The errors for the koff constants are the
standard errors from fitting dwell time distribution. The errors for the kon constants are the
standard errors from average.
65
66
Table 2.2. RFL and GAP binding by RPA and Aro mutants.
kon shows as one phase. koff shows as two phase: koff-fast and koff-slow. koff for one
phase was also shown. The errors for the koff constants are the standard errors from fitting
dwell time distribution. The errors for the kon constants are the standard errors from
average.
67
68
Table 2.3. Representative histograms of RPA and Aromatic mutants binding to different
lengths of ssDNA.
On times or off times were combined to plot histograms, in which the number of
events was plotted against the times(s). The histograms of on times (left panel) and off
times (right panel) for the indicated proteins and length of DNA were shown. Red line
showing the fit to either a single exponential or two-phase exponential decay is shown for
each histogram.
69
70
71
72
73
Table 2.4. Representative histograms of RPA and Aromatic mutants binding to RFL and
GAP DNA
On times or off times were combined to plot histograms, in which the number of
events was plotted against the times(s). The histograms of on times (left panel) and off
times (right panel) for the indicated proteins binding to RFL and GAP were shown. Red
line showing the fit to either a single exponential or two-phase exponential decay is
shown for each histogram.
74
75
76
CHAPTER 3
SINGLE MOLECULE-BASED ANALYSIS OF CONFORMATIONAL
DYNAMICS OF THE RPA-SSDNA COMPLEX
Abstract
RPA is the central hub protein that coordinates multiple protein assemblies in
different DNA processing pathways, including replication, repair and recombination.
RPA is composed of three subunits that together contain eight functional domains. A
critical feature of RPA is the flexible linkers between the domains. This design makes
RPA a highly dynamic protein. The underlying basis for how structural dynamics of RPA
is correlated with its multiple biochemical functions is unknown. NMR, X-ray scattering,
crystallography and computational approaches have been used to define the structures of
individual domains and to begin to define the dynamics of RPA architecture and its
remodeling as it binds ssDNA. However there is still a gap in understanding how
structural changes in RPA drive function. In my studies, I applied single molecule total
internal reflection (smTIRF) FRET analysis to study the RPA-DNA complexes. Domains
of RPA were fluorescently labeled and binding to fluorescently labeled DNA monitored.
Förster resonance energy transfer (FRET) between the labeled RPA and labeled DNA
provided information about the location of domains in RPA-DNA complex and real-time
conformational changes. We found that RPA-DNA complexes generally have a constant
FRET signal for each binding event. The primary determinant of FRET intensity appears
to be the location of RPA binding along the DNA. My data also suggest that the Nterminal domain of RPA1 (DBD-F) is flexible and can interact with unbound regions of
ssDNA to change the FRET signal in the complex. We also propose that domains of RPA
might undergo microscopic dissociation without affecting the global RPA-DNA
structure.
77
Introduction
RPA is essential for DNA replication and repair. RPA binds with ssDNA with
high affinity (Kd~0.05 nM). The high affinity binding of RPA allows it to immediately
localize to ssDNA intermediates exposed during metabolic processes involving DNA.
RPA also interacts with other proteins and acts as a scaffold for protein assembly on
ssDNA. This is thought to help modulate incoming proteins to promote efficient DNA
replication and repair.
RPA is composed of three subunits, RPA1, RPA2 and RPA3(6) with seven
structured domains connected by flexible linkers (26). Each of the RPA subunits contains
one or more OB folds commonly referred as DNA-binding domains (DBD) (26). DBDs
are designated with letters A-F. RPA1 contains four DBDs (DBD-F, A, B and C; Figure
1). RPA2 is composed of two structured domains: a central DNA binding domain (DBDD) and a C-terminal winged helix domain (wh). RPA3 is composed exclusively of an
OB-fold (DBD-E) that interacts weakly with DNA. The three subunits of RPA form a
very stable complex with one DBD in each subunit interacting to form the trimerization
core (DBD-C, -D, -E) (27). All the other parts of RPA extend from the trimerization
domain on flexible protein linkers. The flexible, often long, unstructured linkers allow the
other domains in RPA to rotate independently and to adopt a variety of conformations
(28). Structural studies have shown that four DBDs interact with ssDNA to form a stable
complex with ~30 nt of ssDNA (28,46). RPA binds to ssDNA with specific polarity, with
domains A through D binding from the 5’- to the 3’-end of a given sequence in a
complex (48,154).
RPA binds ssDNA with at least two binding modes (3). A low affinity-binding
mode has an occluded binding site of ~8 nt and a dissociation constant (Kd) ~50 nM,
referred to as the 8 nt mode (33,51). The other, high-affinity mode has an occluded
binding site of ~30 nt and a Kd of 0.05 nM, termed the 30 nt mode (32,50). The high
affinity binding mode is thought to involve all four DBDs, while the low-affinity mode
78
involves only DBD-A and DBD-B (49). By switching between low and high-affinity of
RPA binding modes, the two modes are thought to reflect initial binding of RPA to
ssDNA and the displacement of RPA by other factors during processing (3,46).
RPA function is regulated by protein-protein interactions and posttranslationalmodifications (3). It has been proposed that these regulatory interactions change RPA
conformation to alter the ssDNA-binding properties of RPA (3) by changing the
arrangement of DBDs and the structure of the linker regions in between (46). This can be
achieved by modulating the association of individual DBDs in RPA. Studies have
showed that hyperphosphorylated RPA2 N terminus competed with ssDNA to bind to the
basic cleft of DBD-F or binding cleft of DBD-B to inhibit RPA binding to ssDNA
(116,118).
Recent studies by Nguyen et al and Gibb et al suggested that RPA interactions
with DNA are highly dynamic and that interactions of individual DBDs may contribute
significantly to RPA functions (122,151). They showed that RPA can diffuse along the
ssDNA to melt the secondary structure and can be displaced from ssDNA by other
ssDNA-binding proteins in a concentration-dependent manner. These properties are
thought to arise as a result of microscopic dissociation and association of individual
domains with DNA.
The challenge to the field is that understanding the dynamics of these complexes
will require defining the molecular interactions of individual domains and conformation
changes in RPA-DNA complexes in real-time. My current studies address this problem
by using single molecule TIRF microscopy to analyze the relative positions and dynamics
of fluorescently labeled RPA binding to different forms of fluorescently labeled DNA.
I have generated RPA with fluorescent labels on either DBD-A or DBD-F and
biotin label on RPA3 (called RPA-Cy5A and RPA-Cy5F, respectively; Figure 3.1B and
C). I then tether RPA to a slide and measure the binding of different forms of Cy3
labeled DNA in smTIRFM (see schematic in Figure 3.3 A and 3 C). FRET between Cy3
79
and Cy5 causes a red signal when Cy3 is excited. The strength of the FRET signal
depends on the distance and geometry between the two labels. Real-time changes in the
FRET signal indicate changes in distance and/or geometry.
In the case of RPA binding to ssDNA a number of factors theoretically could
affect the FRET signal produced (Figure 3.3 B). FRET will depend on the position of the
labels on the DNA and the RPA, and the conformation(s) of the RPA complex. The
length of the DNA and the position along the DNA at which RPA is bound will also
influence the FRET signal. Finally, microscopic dissociation of individual domains
would be predicted to affect FRET. The other factor that is critical is the time scale of
any changes. In my experiments, fluorescence is measured every 100 mSec. So
conformational changes or dynamics on shorter time scales will be averaged or not
observed in my experiments.
The studies presented here showed that RPA binds to different positions along the
DNA (giving different FRET signals) and that the complexes formed remain fairly
“ridged” for the duration of the binding event. (Or that any conformational changes in the
complex are faster than the resolution of the experiments, <100 mS.). I also have
evidence suggesting that domains of RPA undergo microscopic dissociation without
changing the FRET of the RPA complex. With certain RPA and DNA combinations,
some FRET changes were observed. The duration and rate of FRET changes observed
suggest some of the complexes undergo a conformational change. My current model is
that these FRET changes are caused by transient interactions between the regulatory
DBD-F domain and the DNA.
80
Materials and methods
Constructs for expression of aldehyde tagged-DBD-F,
DBD-A and DBD-C
Plasmids containing FGE insertions, FGE-DBD-F, FGE-DBD-A and FGE-DBDC, were ordered from Genescript. These contain the DNA sequence for the indicated
domain of RPA1 with the six amino acid FGE (Formylglycine generating enzyme)
recognition signal (LCTPSR) inserted in a surface loop near the DNA binding site in the
domain. The site of insertion was: DBD-F at G36-G37, DBD-A at S215-S216 and DBDC at E534-S535. The modified domain coding sequences containing the FGE site from
FGE-DBD-F, FGE-DBD-A and FGE-DBD-C were then cloned into p11d-biotin tRPAFSPN. This plamids was made by inserting the biotin RPA3 gene from pUC57 BiotinRPA3 on a SacI and BamHI fragment into p11d-tRPA-FSPN. (p11d-tRPA-FSPN
contains all three genes of RPA under the control of the T7 promoter.) First the wildtype RPA3 gene removed from p11d-tRPA-FSPN by BamHI digestion. Then a SacI
restriction site was introduced between BmtI and BamHI sites of the p11d RPA FSPN
with RPA3 removed. The DNA insert that contained the SacI site was annealed from two
oligos: 5’/5Phos/CGC AAGACCAGAGCTCGAGAAGCGGTCATGAGCACCTG3’and 5’/5Phos/GATCCAGGTGCTCATGACCGCTTCTCGAGCTCTCCTCTTGCGCTAG-3’
and inserted into p11d-tRPA-FSPN cut with BmtI and BamHI. In this plasmid (p11dbiotin tRPA-FSPN) the RPA1 sequence has FseI, SalI, PmlI, NotI sites between at the
beginning of the F-A linker, the end of the F-A linker, between A & B, between B & C,
respectively. There is also unique SfiI and BmgBIs site at the beginning and end of the
RPA1 coding sequences. The modified DBD-F, DBD-A and DBD-C domains were used
to replace the corresponding wild-type domain using SfiI-SalI, SalI-PmlI and NotIBmgBI sites, respectively. This yielded expression constructs that have an FGE site at
81
DBD-F, DBD-A or DBD-C of RPA. This produced three plasmids: p11d-RPA-FSPNbiotin FGE-F, p11d-RPA-FSPN-biotin FGE-A and p11d-RPA-FSPN-biotin FGE-C.
Each expresses aldehyde tagged RPA1 (with the tag in the indicated domain).
DNA oligonucleotides
All DNA oligonuclotides are ordered from IDT. The list of oligonucleotides
include dT35 Cy3 5’ end labeled, dT35 Cy3 3’ end labeled, dT20 Cy3 5’ end labeled,
dT66 Cy3 5’ end labeled. The partial duplex substrate that contains both Cy3 and Cy5
dyes and a 42nt of ssDNA are annealed from:
5’CGTACTGCAATCTTGAACCG(T)20/Cy3/GGAATTAAGCTCTAAGCCATCC 3’
and 5’ /Cy5/CGGTTCAAGATTGCAGTACG 3’.
Protein purification of aldehyde-tagged RPA
The purification procedure is same as previously described for purifying nonbiotinylated RPA, with the exception that 0.2% arabinose (Sigma) was added 30 minutes
before adding IPTG to induce expression of formyglycine generating enzyme (in
pRSFFGE), which was co-transformed with the individual p11d-RPA-FSPN-biotin FGE
plasmids. The yield of DBD-A and DBD-F modified complexes was approximate 1/3
that for wild-type RPA. The DBD-C modified complex had an even lower yield and was
not worked with further.
Labeling aldehyde-tagged RPA
The purified aldehyde tagged RPA was concentrated to 20 µM-40 µM and
exchanged into the labeling buffer containing (250 mM potassium phosphate (pH 7), 500
mM KCl, 5 mM DTT) by using the Amicon Ultra (0.5 ml) Centrifugal Filter 10K. Then,
3 µl of concentrated protein was mixed with 0.1 mg of Cy5 hydrazide (GE Healthcare)
and incubated in darkness at 4 C° for 24 hour in a rotator. Bio-Spin 6 (Bio-rad) was used
to remove free dye and labeling buffer was exchanged to storage buffer (HI-0 buffer, 300
82
mM KCl). The labeling efficiency was measured by UV-vis spectrum. The absorption of
protein and Cy5 were measured at 280 nm and 643 nm, respectively.
Single-molecule smTIRF and reaction conditions for the
single-molecule assay
Single-molecule smTIRF was carried out as described in Chapter 2.
smTIRF Data analysis
The single molecule trajectories were extracted as described in Chapter 2. The
individual trajectories were visually inspected and picked by using MATLAB.
Trajectories that showed FRET signal or Cy5 signal were analyzed for FRET status. The
signal from Cy3 channel and Cy5 channel was quantified and export to Excel, where
FRET efficiency was calculated: FRET=Cy5/(Cy5+Cy3). The averaged FRET for each
binding event was plotted vs. the number of events.
Results
Fluorescence labeling of RPA for single-molecule imaging
Because of the flexible structure of RPA, the direct observation of domain
arrangements upon binding DNA is difficult. Therefore, single molecule analysis was
utilized to characterize the complex(es) formed when RPA binds to DNA. DBD-F and
DBD-A of RPA1 were labeled with Cy5 and the interactions between labeled domain and
Cy3 labeled-DNA were analyzed. To label RPA at specific site, we used the FGE
labeling system, which allows site-specific protein modification using a genetically
encoded aldehyde tag (155). The peptide sequence (LCTPSR) recognized by
formylglycine generating enzyme (FGE) was incorporated at DBD-F (inserted between
G36-N37), DBD-A (S215-R216) (Figure 3.1 A). These modified coding sequences were
then inserted into a plasmid expressing RPA2 and biotinylated RPA3. Co-expressing of
FGE in cells will converts the cysteine in the peptide sequence to formylglycine,
83
producing RPA with a single aldehyde group. The purified aldehyde-tagged RPA was
then incubated with Cy5 hydrazide that specifically reacts with the aldehyde group to
label RPA with Cy5 at that site and no other. The Cy5 labeled RPA at DBD-F and DBDA was confirmed by running the labeled protein on an SDS PAGE gel (Figure 3.1 B).
The labeling efficiency measured was estimated to be ~30% (comparing absorbance at
280 and absorbance of Cy5).
To determine whether the modification has change RPA binding activity, the
binding activity of labeled-RPA complexes was compared to the non-labeled RPA using
smTIRF. However, there were too few trajectories observed to calculate Ka for the
labeled RPA. It is likely that this is because of several factors including (1) the low
efficiency of labeling, (2) some loss of active RPA during labeling process and (3)
bleaching/loss of the Cy5 dye during experiments. To address this, I did controls with
unlabeled, aldehyde-modified RPA. The obtained kon, koff, and the Ka for the aldehydemodified RPA complexes binding to different DNA substrates (dT66, dT35 5’, dT35 3’
and dT20) are summarized in Table 3.1. We compared the kon, koff and Ka of modified
RPA to that of wild-type RPA. My previous studies (Chapter 2) had shown that that the
“off times” of RPA binding fit best to a one phase-exponential decay, and the “on times”
fit best to a two-phase exponential decay. The aldehyde-modified RPA had similar “on
times” and “off times” distribution fitting to a two-phase exponential decay. RPAaldehyde-DBD-A and RPA-aldehyde-DBD-F had the similar Ka for dT35 and dT20 as
wild-type RPA (Table 1). Overall, the affinities of modified complexes are similar to
those of the unmodified complexes (Figure 3.2). The fitting of RPA-Cy5A to dT20 fit to
different kinetics suggesting that there were modest changes in the binding with RPACy5A to short ssDNA. RPA-Cy5F also had different kinetics that showed faster on and
off rates. The later two points indicate that the labeling position could be affecting the
interactions of the labeled domain. Since aldehyde-modified RPA binds to dT35 and
dT20 with high affinity and has similar binding affinity to the non-labeled RPA, we could
84
use FRET data obtained from the labeled RPA to give us useful insights into the domain
location and arrangements of RPA binding.
RPA binds to different positions along the DNA
The FRET status for each binding event gives information on the relative distance
between DNA and labeled domain. To determine the location of DBD-A and DBD-F
relative to the 5’ end of DNA, the binding trajectories of RPA labeled on DBD-F and
DBD-A obtained with different lengths of Cy3 5’ end labeled DNA (dT66, dT35, dT20)
were analyzed (Figure 3.3 C). The FRET signal of each binding event was determined.
My experiments showed that RPA-Cy5A binding to Cy3 5’ labeled dT35 gave a range of
FRET signals with some high (FRET >.8), medium (.4< FRET <.8) and low (FRET <. 4)
signals (Table 2). In contrast, RPA-Cy5A binding to Cy3 5’ labeled dT20 gave only high
or medium FRET signals. When RPA-Cy5A bound to 5’ labeled dT66, only medium and
low FRET signals were observed. These results suggested that DBD-A was located at
different distances from the 5’ end of the DNA in different binding events. With longer
ssDNA, DBD-A is more likely to bind away from 5’ end of DNA so DBD-A is generally
closer to the 5’ end of the DNA with short oligonucleotides. I concluded that the primary
determinant of FRET intensity in these complexes is the position of RPA binding along
the DNA (Figure 3.7 A).
We also examined complexes containing RPA with DBD-F labeled with Cy5.
DBD-F is linked to DBD-A by a long flexible linker and current evidence suggests that it
is sampling a large number of conformations on a sub-µS time frame (Figure 3.7 B).
RPA-Cy5F interactions with Cy3 5’ labeled dT35 gave only medium and low signals
(Table 3). While with RPA-Cy5F binding to Cy3 5’ labeled dT20 individual binding
events ranged from high to low FRET (Table 3). When RPA-Cy5F bound to 5’ labeled
dT66, only medium and low FRET signals were observed. These data are consistent with
the previous studies with RPA-Cy3A, suggesting that there are more binding options and
85
more complexes with low FRET with longer ssDNA fragments. The differences in
FRET distributions between RPA-Cy3A and RPA-Cy3F suggested that DBD-F is
generally located farther from the 5’ end of the DNA compared to DBD-A. It is most
likely that the FRET signals are the result of an averaging of the possible conformations
of DBD-F.
RPA has multiple DBDs that interact with ssDNA. The stable 30-nt complex
contains 3 or 4 DBDs interacting with the DNA. In contrast, less stable complexes are
thought to have only DBD-A and -B binding. The position along ssDNA that RPA binds
relative to the 3’ and 5’ ends will determine the number of DBDs that can associate with
the ssDNA. So one possible explanation for the low FRET complexes is that they formed
when RPA binds close to the 3’ end of the DNA. If this were the case, low FRET
complexes would also be expected to have fewer DBDs binding and these complexes
should be less stable. This predicts that low FRET complexes would have shorter dwelltimes and medium or high FRET complexes would be more stable and have longer dwelltimes. However, when the length of the binding events were plotted versus the FRET
signal, we found that the intensity of FRET was not correlated with the length of binding
event (Table 2 and Table 3; shown as dwell-times (s) as function of FRET). (i.e. There
was not a strong a correlation between the length of the binding event and the strength of
the FRET signal.) This suggests that all of the complexes observed have a similar
stability and that probably four DBDs are interacting with the DNA in both high and low
FRET state complexes.
RPA binds with 5’-3’ polarity and adopts a less dynamic
and condensed structure on binding ssDNA
Previous studies indicated that RPA binds ssDNA with a defined polarity; DBDA and -B (the high affinity-binding domain of RPA) are positioned at the 5’ side of the
complex while the weaker ssDNA-binding domains (DBD-C and -D) reside at the 3’ end.
86
Thus, DBD-A is expected to be located farther from 3’ end than from the 5’ end. So next
I examined the FRET distribution of RPA-Cy5A and RPA-Cy5F binding to Cy3 3’
labeled dT35. RPA-Cy5A-3’labeled dT35 complexes had mostly medium and low FRET
(Table 2). (Though there were a small number of high FRET events observed with 3’
labeled dT35). This is consistent with DBD-A located farther from 3’ end of DNA and
RPA the known polarity of RPA. The finding that medium FRET signals were observed
suggests that DBD-C is located within FRET transfer distance of DBD-A (<10nm) in the
complex. This is consistent with RPA adopting a compact complex upon binding to
ssDNA.
I also looked at RPA-Cy5F binding to Cy3 3’ labeled dT35. RPA-Cy5F
complexes also had mostly medium and low FRET signals. However, the distribution
had two distinct features. There were a large number of events with very low FRET
signals. The remaining events observed had a distribution similar to the complexes
formed with Cy3 5’ labeled dT35 and dT20 and included some high FRET events (Table
3). This is suggested that (i) that DBD-F is generally farther from the 5’ end of DNA
than DBD-A (ii) in most complexes, DBD-F is located a similar distance from both the 5’
end and 3’ ends of DNA, and (iii) that in a small number of complexes DBD-F is close
enough to the 3’ end of the DNA to give a high FRET signal. DBD-F is located at the
end of a long flexible linker. These findings are consistent with DBD-F being can adopt
multiple conformations in the RPA-DNA complex.
The flexible DBD-F domain contributes to the FRET
changes in complex
In most experiments, the FRET signals were stable throughout each binding event
(Summarized in the column in Table 2 and Table 3.) The two representatives trajectories
that have stable FRET signal are shown in Figure 3.4. The lack of dynamic changes in
FRET in RPA-DNA complexes suggested that under my conditions, RPA was not
87
undergoing conformational changes on a 100 mS time scale. This suggests that diffusion
along the DNA was not occurring and that any changes in individual domain interactions
were not affecting FRET of the complex.
Interestingly, there were binding events with RPA-Cy5A and RPA-Cy5F binding
to Cy5 5’ 3 dT66 and Cy3 3’ dT35 that showed FRET changes (Figure 3.5 A). 20 out of
144 binding events and 8 out of 100 binding events with RPA-Cy5A binding to Cy3 5’
dT66 and Cy3 3’ dT35 that had changes in the FRET signal during the binding event
(Table 2 and Table 3). Also, 7 out of 52 binding events and 24 out of 107 binding events
with RPA-Cy5F binding to Cy3 5’ dT66 and Cy3 3’ dT35 that showed changes in the
FRET during the binding. This suggested that there is some type of conformational
change(s) in some these complexes.
With Cy3 3’ labeled dT35, 8 out of 100 events with RPA-Cy5A and 24 out of 107
events with RPA-Cy5F showed FRET changes during binding. No FRET changes were
observed with Cy3 5’ labeled dT35. I conclude that DBD-A and DBD-F changed
position with respect to the 3’end of the labeled DNA in some complexes with dT35. In
both of these complexes, the labeled end of the DNA is far away from the 5’ end of the
RPA-DNA complex (i.e. DBD-A) (Figure 3.7 C).
In the case of Cy3 5’dT66, most complexes will have substantial free DNA at the
5’ end of the DNA (Figure 3.7 C). It seems most likely that this free DNA is contributing
to the changes in FRET. I predict that the motions of the free DNA will be rapid on the
time scale of these experiments so that the change in FRET represents distinct
conformations or slow motions in the complex. In the case of Cy3 3’dT35, there is less
free DNA ssDNA and the change in FRET must reflect the labeled domain coming close
to the 3’ end of the DNA (Figure 3.7 C).
There were fewer dynamic complexes for RPA-Cy5A•3’dT35 compared to RPACy5F•3’dT35. DBD-A binds stably to the 5’end of the DNA while DBD-F connects to
DBD-A through the long linker and is located outside of the binding core. So it is likely
88
that the stably associated DBD-A is less accessible to the 3’ end of DNA compared to
DBD-F. Nevertheless, in both cases, some of the complexes were undergoing a
conformational change that caused the two labels get closer in space (Figure 3.7 C).
The evidence of microscopic dissociation within RPADNA complex
Besides looking at the spatial localization of domains in RPA-DNA complex, we
also wanted to determine whether RPA binding had any effects on the bound DNA. So I
next examined the RPA binding to a partial duplex DNA which contained both Cy3 and
Cy5 labels, as shown in Figure 3.6 A. This DNA structure has an 18 bp duplex region
followed by 42 nt ssDNA. There is 20 nt ssDNA between the two dyes. This DNA was
incubated with unlabeled biotin-RPA and observed by smTIRM. Binding of this DNA
resulted in binding events that generate FRET signals. In most binding events, a medium
FRET signal was observed in the DNA (Figure 3.6 B). However, there were few binding
events in which no FRET was observed. It is most likely that these events arise from
DNA that has melted, though I cannot rule out an alternative binding conformation.
I expanded the scale to examine the dynamics of the DNA in the RPA-DNA
complex. I observed large fluctuations of the FRET signal in the RPA-DNA complex
(Figure 3.6 C). This suggests that the orientation and/or distance between the dyes was
changing on the 100 mSec time scale. One possible cause of the observed dynamics
within RPA-bound DNA is the microscopic dissociation of RPA domains as has been
observed previously (54).
Discussion
RPA plays a central role in DNA processing, but little information is available on
the physical basis by which RPA coordinates different functions. Because of the modular
nature of RPA, it has been difficult to obtain the global structure of the full length RPA
interacting with DNA. Learning the position of RPA subunits on ssDNA is likely to
89
provide insights into the structural changes and domain arrangements that occur upon
binding to ssDNA. Here, I have been able to characterize the molecular interactions of
individual domains and the conformation changes in RPA-DNA complexes in real-time
by using smTIRF. My results are consistent with the known properties of RPA. DBD-A
has the highest affinity to DNA and stably associates with 5’ end of DNA. DBD-F has a
weak DNA binding affinity and has been suggested to regulate the complex.
Recent studies have indicated that RPA binding to ssDNA is dynamic and that
RPA can diffuse along ssDNA and be displaced from ssDNA by other DNA binding
proteins (54,122). In particular, Nguyen and coworkers showed in TIRFM that the Nterminal labeled RPA can diffuse long the surface tethered labeled DNA. By monitoring
the FRET fluctuations, they could see constant movements of Cy5 labeled RPA relative
to the Cy3 at the 3’ end of the DNA. In Nguyen’s analysis, different forms of DNA were
attached to the slide and binding and dynamics were monitored using either doublelabeled DNA (like that shown in Figure 3.6A) or with RPA labeled at the N-terminus of
one of the three subunits. In my analysis, I saw a similar dynamics when I monitored
RPA binding to double labeled DNA (Figure 3.6). The RPA-DNA complex showed
FRET changes from 1 second to 3 second. These results are consistent with Nguyen’s
studies that were interpreted to be the result of microscopic dissociation and/or domain
movements that bring the two dyes together inside the complex.
On the other hand, a recent structure study of RPA showed that RPA has a
dynamic but condensed structure when binds to DNA (52). The domains of RPA are
structurally independent and occupy a range of inter-domain orientations in solution
(33,156). However, the binding of DNA limits the inter-domain interactions of RPA and
makes the RPA complex become progressively condensed and less dynamic (28,52,156).
My studies examining interactions of labeled RPA with labeled DNA provide additional
insights into the RPA-DNA complex in solution. We showed that DBD-F locates similar
distance to both 5’ and 3’ end of DNA. DBD-A is close enough to 3’ end of DNA to
90
generate medium and high FRET signal. This suggested that RPA undergoes compaction
upon binding to ssDNA. In agreement with this condensation of RPA complex upon
binding ssDNA, we observed that the complex was fairly ridged for duration of binding
events. In most cases we did not observed FRET changes that would indicate domain
arrangements on a ~100 mS time scale. We also didn’t observe fluctuations in the FRET
signal that would indicate diffusion of RPA on ssDNA. The discrepancy between my
observation and Nguyen’s group could be explained by differences in the tethering used:
Nguyen used tethered DNA while my studies used tethered RPA. However, this does not
seem to be the full explanation because I observed DNA dynamics like those observed by
Nguyen using double-labeled DNA. In addition, Nguyen’ s group used RPA labeled at
the amino termini of three subunits, so position of the Cy5 label was on any (and in some
cases probably multiple) RPA subunits. Because the N-termini of RPA2 is a flexible
linker, the observed diffusion on DNA might be contributed to by rapid movement of the
N-termini of one of more RPA subunits (especially RPA2). Another difference between
my studies and those of Nguyen was that most of their studies used 3’ labeled DNA. So
there may be more dynamics at the 3’ end of the RPA•DNA complex (see below).
My data indicate that the core of the RPA-ssDNA complex does not undergo
conformational changes during binding events (or it undergoes conformational changes
that do not cause a change in FRET). Instead, it appears that the primary factors that
influence the FRET signal is the location of the labels and position on the DNA where
RPA binds. This suggests a model in which RPA interacts with a random position along
ssDNA (Figure 3.7 A). This interaction is primarily diffusion limited (see chapter 2). It
seems likely that RPA binding to the center or to the 5’ end of DNA will form more
stable complex than if it binds close to 3’ end of DNA. However, we didn’t see any
correlation of length of binding and strength of FRET signal. This suggests that RPA
binding is not just sequential association of DBDs with an initial interaction between
DBD-A and the 5’ end of DNA followed by association of other DBDs. So my results
91
are consistent with the model that the DBDs of RPA are structurally independent and that
binding could be initiated by any of the DBDs. Further, the FRET signal for binding
events almost always remained constant. This indicates that the RPA-DNA complex
does not undergo significant conformational changes during binding.
In a minority of complexes with certain DNAs, I did observe evidence for a
conformational change. Some complexes of RPA-Cy5A and RPA-Cy5F bound to Cy5 5’
3 dT66 or Cy3 3’ dT35 showed multi-second dynamic FRET changes. Based on the other
complexes that did not show FRET changes, it seems unlikely that these changes
represent an alternative mode of binding or a major change in the conformation of the
DNA-binding core of RPA. All the dynamic binding events involved either long ssDNA
(dT65) or the 3’ end of the DNA (Cy5 3’ end labeled dT35). The FRET changes in
individual binding events had durations from several seconds up to 40 seconds. This
duration limits the type of conformational change that could be causing these dynamics.
Previous studies have shown that the time scale of ssDNA conformational changes are
very fast (157). Similarly, DBD-F is predicted to move on the sub-µS time frame. So it
is unlike that either random conformations of the un-bound regions of DNA or DBD-F
are causing the multi-second alterations in FRET in these complexes. It also seems
unlikely that diffusion of RPA along the DNA could cause these changes because they
appear as distinct events rather than the random walk that should be caused by diffusion.
This leaves two likely causes of these FRET changes: microscopic changes in the
interactions of individual domains or conformational changes in the complex (or both).
Further, the specific RPA-DNA pairs that show dynamic complexes suggest that the
FRET changes were caused by transient interactions between DBD-F and free DNA ends
(Figure 3.7 C).
My results suggested that DBD-F and DBD-A could get close to the 3’ end of
DNA, which will be interacting with, or be close to, DBD-D. Because our data indicates
that DBD-A remains stably associated with DNA for the duration of binding, We propose
92
that it is most likely that there is microscopic dissociation of the weaker binding DBD-C
and DBD-D in these complexes. However, microscopic dissociation of domains might be
so fast that would be undetectable under my experimental condition. The microscopic
dissociation of domains needs to be further investigated and confirmed with using the
tethered DNA.
Structural studies in the presence and absence of ssDNA have shown that DBD-F
interacts weakly with ssDNA and remains autonomous from DBD-A and DBD-B even
when the latter two domains bind ssDNA (158-161). My results are consistent with DBDF adopting multiple conformations in the RPA-DNA complex. Moreover, because of the
flexibility nature of DBD-F (and it not being part of the core DNA complex), it is likely
that DBD-F could sense either a free 5’ end or a dissociated 3’ end of DNA compared to
DBD-A. Thus, my preferred model is that in all the cases where dynamic FRET is
observed, DBD-F is interacting with the end of the DNA and causing the two labels to be
in close proximity for the duration of the interaction (Figure 3.7 C).
In the future, I would like to examine binding to other single stranded and
partially duplex DNAs. I would also like to label the other domains in RPA and try
positioning the labels in different locations on the same domain. This will help define
both the conformations of the complex and how the conformation changes when RPA
binds to different DNA structures. I would also like to label two domains of RPA with
different dyes in the same experiment. This will allow us to directly observe the
structural changes that occur of RPA with binding to DNA.
93
Figure 3.1.Site-specific modification of RPA at DBD-F and DBD-A.
(A) A schematic representation of RPA1 subunit with the location of the peptide
sequence insert shown. (B) The purified, labeled DBD-F and DBD-A forms of RPA
(biotin RPA-Cy5F and RPA-Cy5A) were separated on an 8-14% SDS-PAGE. The same
gel was stained with silver and visualized by fluorescence. The gel image was taken
under a Cy5 channel camera to detect the Cy5-labled protein. The locations of three RPA
subunits are shown. RPA and biotin RPA were loaded as control. (C) Schematic
representation of RPA labeled on DBD-A and DBD-F.
94
A
B
C
95
Figure 3.2. Aldehyde modified RPA complex showed similar high binding affinity to
non-modified RPA complex.
The equilibrium Ka obtained from smTIRF for unlabeled RPA-Cy5A and RPACy5F binding to dT35 and dT20 was compared to RPA.
96
Figure 3.3. The FRET status of RPA-DNA complex with RPA-Cy5F and RPA-Cy5A in
smTIRF.
(A) Schematic representation of RPA-DNA complex. RPA binds ssDNA
directionally, with DBD-A through DBD-D binding from the 5’ to the 3’ end of a given
complex. A representation of RPA-Cy5A interacting with Cy3 5’ labeled dT35 is also
shown. The FRET signal can be obtained from the interaction of the fluorophores on the
RPA and the DNA (purple arrow). (B) Schematic representation of possible factors that
influence FRET in an RPA-DNA complex. From left to right: DNA length, location of
binding, position of labels, RPA conformation and domain dissociation. (C)
Representative single molecule trajectories of RPA-Cy5F and RPA-Cy5A binding to Cy3
labeled DNA.
97
A
B
C
98
Figure 3.4. The RPA-DNA complex remains fairly rigid for the duration of binding
events.
The representative trajectories of RPA-Cy5A and RPA-Cy5F bind to Cy3 5’
labeled dT35. A zoom-in image of one of the FRET events is shown. The FRET intensity
is plotted as function of time (S) (blue line).
99
100
Figure 3.5. Representative dynamic complexes observed with RPA-Cy5A and RPACy5F binding to Cy3 3’ labeled dT35 and Cy3 5’ labeled dT66.
(A) Representative trajectories of RPA-Cy5A and RPA-Cy5F that showed FRET
changes. (B) The FRET changes for the representative dynamic binding events was
plotted as length of time (blue line).
101
A
B
102
Figure 3.6. DNA bound by RPA exhibits FRET dynamics during binding events.
(A) Schematic representation of partial duplex and double-labeled DNA substrate.
(B) Representative trajectory of RPA binding to this substrate shows FRET binding
event. (C) The zoom in image of one of the FRET binding events is as shown. The FRET
is plotted as function of the time (s) (blue line).
103
A
B
C
104
Figure 3.7. The proposed model of RPA-DNA complex based on observed FRET states.
(A) RPA binding position along the ssDNA results in different FRET intensities.
DBD-A location close to 5’ end gives high FRET. DBD-A location away from 5’ end
gives low FRET. (B) Fast motions in the RPA-DNA complex. (C) The model of dynamic
RPA-DNA complex. The schematic representation of dynamic RPA-Cy5A binding to
Cy3 5’ labeled dT66 complex (right) and RPA-Cy5F binding to Cy3 3’ labeled dT35
(left).
105
A
C
B
106
Table 3.1. RPA-Cy5A and RPA-Cy5F bind to DNA substrates with the same high
affinity as RPA. The equilibrium constant is measured by smTIRF.
Substrate kon (108M-1s-1) koff (s-1)
Ka (M-1) koff-fast (s-1) koff-slow (s-1) Percent fast Kafast (M-1)
Kaslow (M-1)
2.24 ± 0.58 109
1.90 ± 0.01 109
1.82 ± 0.01 109
1.71 ± 0.06 109
0.25 + 0.02
0.40 + 0.04
0.37 ± 0.05
0.50 ± 0.09
0.30 + 0.04
0.21 + 0.01
0.26 ± 0.02
N/A
0.02 + 0.01
0.05 + 0.01
0.04 ± 0.01
0.03 ± 0.01
0.03 + 0.01
0.01 + 0.01
0.02 ± 0.01
N/A
83% + 1%
60 % + 11%
67 % ± 3%
77% ± 4%
82% + 3%
91 % + 2%
86 % ± 3%
N/A
1.30 ± 0.12 109
1.03 ± 0.31 109
1.05 ± 0.28 109
7.61 ± 1.29 109
1.34 ± 0.51 109
1.50 ± 0.08 109
1.23 ± 0.06 109
N/A
2.14 ± 0.53 1010
7.83 ± 0.14 109
9.01 ± 1.50 1010
1.10 ± 0.15 1010
1.62 ± 0.80 1010
2.98 ± 1.17 1010
1.41 ± 0.18 1010
N/A
RPA
0.18 + 0.01
0.16 + 0.01
0.17 + 0.01
0.18 ± 0.24
2.18 ± 0.64 109
3.01 ± 0.48 109
2.67 ± 0.30 109
1.62 ± 0.07 109
1.47 ± 0.27 109 1.66 ± 0.59 1010
1.25 ± 0.17 109 1.52 ± 0.31 1010
3.90 + 1.11
3.20 + 0.01
3.20 ± 0.01
3.01 ± 0.29
0.16 + 0.04
0.14 + 0.05
0.14 + 0.01
0.23 ± 0.01
3.20 + 0.14 0.13 + 0.02 2.45 ± 0.23 109 0.22+ 0.05 0.02 + 0.01 82% + 5%
2.50 + 0.14 0.19 + 0.01 1.31 ± 0.14 109 0.21 + 0.02 0.02 + 0.01 98% + 2%
RPA-Cy5A dT66
dT35 5’
dT35 3’
dT20
3.25 + 0.01
4.01 + 0.08
3.80 + 0.06
3.80 ± 0.01
dT35 5’
dT20
RPA-Cy5F dT66
dT35 5’
dT35 3’
dT20
107
Table 3.2.The summary table for FRET distribution of RPA-Cy5A binding to different
labeled DNA substrates.
From left to right: the names of substrates; the schematic representations of RPADNA complex; averaged FRET for each binding events plotted vs. the number of events;
the averaged FRET for each binding event was plotted vs. the dwell time of that event;
and the total numbers of binding events for RPA-Cy5A as well as the number of dynamic
binding events that showed FRET change are shown for the indicated RPA-DNA
complexes.
108
109
Table 3.3. The summary table for FRET distribution of RPA-Cy5F binding to the
different labeled DNA substrates.
From left to right: the names of substrates; the schematic representations of RPADNA complex; averaged FRET for each binding events plotted vs. the number of events;
the averaged FRET for each binding event was plotted vs. the dwell time of that event;
and the total numbers of binding events for RPA-Cy5F as well as the number of dynamic
binding events that showed FRET change are shown for the indicated RPA-DNA
complexes.
110
111
CHAPTER 4
DISSCUSION
Overview of findings
RPA is essential for all aspects of DNA metabolism that involve ssDNA
intermediates, including replication and repair. Both processes require RPA binding but
my data has revealed that replication and repair pathways depend on different RPA-DNA
interactions. In replication, RPA binds transiently to protect ssDNA, prevents formation
of secondary structure and facilitates protein assemblies at replication forks. In DNA
repair, RPA localizes to sites of DNA damage which usually contains short ssDNA
regions and must bind with sufficient stability to the (usually short) ssDNA region to
recruit and position repair proteins. My studies have extended previous studies from the
Wold lab that the high affinity-binding domain of RPA (DBD-A and -B) is essential for
RPA function in repair. Specifically, I have shown that the conserved aromatic residues
in DBD-A and -B are needed for RPA to form long-lived RPA-DNA complexes with
short ssDNA. The melting activity and the ability to form long-lived complexes are
required for RPA function in DNA repair but not replication. My studies also showed
that RPA binding involves multiple states either through domain interactions or
conformational changes. Analysis of the conformation and dynamics of RPA-DNA
complex suggested that RPA forms relatively rigid complexes with ssDNA but that there
are some 100 mS-time scale dynamics in some complexes. My data also suggests that
some domains in RPA may be undergoing microscopic dissociations in the complex. I
postulate that these dynamics help allow RPA to function as the central hub in the
processing of ssDNA intermediates.
112
RPA-ssDNA interactions mediated by the conserved
aromatic residues are essential for cellular processes
Forms of RPA with mutations in the conserved aromatic residues in the high
affinity binding sites (Aro mutants) can support DNA replication but not DNA repair. By
studying these Aro mutants, I discovered that RPA has two kinetic states when it binds
ssDNA and that formation of the more stable state seems to be necessary for RPA to
function in DNA repair.
RPA is essential for replication. RPA assembles on the ssDNA immediately after
the helicase unwinds the DNA at the origin and plays a key role in the assembly of
replication forks. At elongating forks, binding of RPA protects ssDNA from nucleases
and prevents hairpin formation (that might inhibit the progression of fork). In addition,
RPA also coordinates with replication proteins at the replication fork to stimulate their
activity. Previous studies have shown the Aro mutants are able to support DNA
replication. My data indicated that mutation of the aromatic residues did not change the
binding parameters for 35 nt ssDNA. Since the replication fork proceeds at high speed
(100 nt/sec), the relatively long (~100-200) ssDNA intermediates in replication only need
to be bound by RPA for short times. So it is not surprising that the high affinity binding
of the Aro mutants to long ssDNA is sufficient for them to function normally in
replication.
RPA also plays an important role in DNA damage response, which includes
activation of checkpoints and stimulation of DNA repair. The localization of RPA to the
sites of DNA damage is one of the signals that trigger activation of ATR and ATM and
eventually leads to cell cycle arrest by checkpoint activation. The presence of RPA is also
essential for repair of DNA damage. Although the role of RPA differs in detail in
different repair pathways, in general RPA binds to and protects ssDNA and then acts as a
recruiter of repair proteins. The key differences between DNA replication and repair are
that in DNA repair, RPA is usually one of the first proteins to bind to a site of DNA
113
damage and RPA must remain bound for long enough for the recruitment and assembly
of the needed repair complex. Also in repair RPA often has to bind to small single
stranded regions that form at sites of damage prior to damage processing or excision. For
example in nucleotide excision repair (NER), RPA is part of the initial damage
recognition complex and without RPA, the endonuclease XPG and XPG/ERCC1 are not
able to localize to the damage site and excise the damage (which creates a ssDNA gap)
(162). Similarly, in double-strand break (DSB) repair, RPA binds early to the break and
coordinates resection of the 5’ end (which creates long single stranded 3’ overhangs). In
both pathways the initial ssDNA region is very short. In NER, the distortion of the
duplex caused by the DNA is recognized and the initial complex is an ssDNA bubble
with RPA bound to the undamaged strand. In DSB repair, the ssDNA intermediates
present at broken end are also initially very short.
Previous studies of the Aro mutants showed that they are defective in both NER
and DSB repair pathways and suggested that these mutants affected binding to short
ssDNA regions. My studies found that mutations of aromatic residues prevented the
stable binding to 15 nt ssDNA. I conclude that the aromatic residues are essential for the
high affinity-binding domain of RPA to stably associate with short ssDNA.
This
suggests that the Aro mutants are unable to participate in DNA repair because they can’t
form the initial stable complexes needed with short ssDNA.
Cells expressing Aro mutants showed accumulation of DNA damage resulting in
checkpoint activation in the absence of exogenous DNA damage. It’s been speculated
that the presence of Aro mutants prevent basal repair of damage and leads to
accumulation of DNA damage. For example, because of the endogenous replication
stress, replication forks that encounter obstacles can stall and collapse into DSBs.
Because Aro mutants are defective in DNA repair, there may be an increased incidence
of fork stalling or collapse with cells expressing these mutants. Replication forks stall
114
sites of DNA damage and stable binding of RPA is needed at a stalled fork for damage
bypass or fork restart.
Recent studies have shown that RPA can be rapidly displaced from ssDNA in the
presence of free ssDNA-binding proteins, such as RPA, Rad51 and E.coli SSB (122). It
has been proposed that domains of RPA undergo microscopic dissociation that allows
other proteins to gain access to the exposed small section of ssDNA. It seems reasonable
to speculate that the Aro mutants may be more sensitive to this type of displacement
because of altered interactions of the mutated high affinity domains. If this is the case
then Aro mutant complexes at stalled forks (or other long-lived ssDNA intermediates)
would be expected to be more sensitive to dissociation and that this would disrupt restart
(or repair).
The high affinity binding of DBD-A and DBD-B are
essential for RPA function
High affinity binding of RPA to ssDNA is a very dynamic process. RPA binds to
ssDNA in two binding modes that differ in the length and affinity of the bound DNA.
The low-affinity mode has an occluded binding site of~8 nucleotides (nt). In this mode,
RPA binding only involves DBD-A and DBD-B. The high-affinity mode has an occluded
binding site of ~30 nt. In this mode four domains are engaged with the DNA (DBD-A, B, -C, and -D). Structure studies also suggest that there is progressive compaction of
RPA architecture induced by progressively longer ssDNA. This flexibility allows RPA
to adapt to different length intermediates and partially duplex structures. It is also likely
that RPA might transition between different binding modes during the processing of
ssDNA intermediates.
Several studies have suggested that RPA forms complexes with ssDNA through
the sequential engagement of the DBD-A through DBD-D domains. In this model, the
binding of the first DBD, most likely the high-affinity DBD-A, increases the effective
115
concentration of the other DBDs at the DNA which then rapidly engage with the DNA to
form the stable, 30 nt complex. While recent studies have suggested that binding is not
sequential, there is no question that the high affinity binding of DBD-A and DBD-B are
essential for the formation of stable RPA-DNA complexes. My analysis of the Aro
mutants in DBD-A and -B highlights this fact. Mutation of the conserved aromatic
residues in DBD-A or DBD-B directly affects binding of these domains and is causing
the repair-defective phenotype.
As discussed above, RPA needs to interact with short ssDNA intermediates and
partially duplex DNA structures for successful repair. RPA also needs to recognize the
distorted DNA or partial duplex DNA structure at sites of DNA damage. This requires
RPA to bind to these DNA structures stably and in some cases promote unwinding the
duplex. RPA mutants with large reductions in the binding of either DBD-A or DBD-B
are not able to support general DNA repair (31,137) or suppress the error-prone microhomology mediated end joining upon DSBs (163). Also, after replication stress, it has
been shown that the high affinity binding of RPA directly affects SMARCAL1-mediated
replication fork remodeling (164). DNA replication stress is defined as inefficient DNA
replication that causes DNA replication forks to progress slowly or stall (165). When the
high affinity binding domains bound close to the fork junction, RPA stimulates
SMARCAL1 activity (164). However, AroA and AroB were defective in stimulating
SMARCAL1 activity. One possible explanation for this observation is that RPA induces
specific DNA conformations by transient destabilizing the fork, which stimulates
SMARCAL activity (164). This study is consistent with my results, which showed AroA,
and AroB are deficient in helix destabilization. Thus, I conclude that high affinity binding
of DBD-A and DBD-B is required for efficient helix destabilization activity by RPA.
116
Aromatic residues and polar residues play different roles in
RPA binding and functions.
The overall affinity of RPA complexes does not directly correlate with RPA
activity. In each binding site in RPA, both polar and aromatic residues have been
identified interacting with ssDNA (49). The polar residues interact directly with the bases
and/or with the phosphate backbone, while the aromatic residues stack between bases.
The polar residues are less conserved than aromatic residues. The interacting aromatic
residues in RPA are conserved from human to yeast and are found in all structurally
similar DNA binding OB folds within the RPA complex. Surprisingly, mutations of
aromatic residues did not have a large effect on the affinity of RPA compared to the polar
residues (32). Forms of RPA with two polar residues mutated in the DNA-binding site of
DBD-A or DBD-B had a lower affinity for ssDNA than either AroA or AroB. However,
these polar residue mutants were fully active while the Aro mutants were completely
defective in DNA repair. My studies suggested that interactions made by aromatic
residues are important for positioning and stabilizing the high affinity-binding domain
and formation of more stable RPA-DNA complexes.
Independent but coordinated RPA domains and
nonequivalent function of Aromatic residues
The four aromatic mutants analyzed have different pairs of residues mutated
either at the same domain or different domains. Four aromatic residues are not equivalent
for their contribution to RPA ssDNA binding. It was found that these mutants range from
being the non-functional (Aro1), to severely defective (AroA, AroB) to mildly defective
(Aro2). So it appears that the aromatic residues are not equivalent. Mutation of both
aromatic residues in the same DNA binding domain seems to have a large effect on the
binding activity of that domain. The nonfunctional Aro1, which has mutations of
aromatic residues F238 and W361, lost binding activity and the mutations partially
117
perturbed the folding of RPA1(137). Examining of the crystal structure of high affinity
binding domains indicated that residues F238 and W361 are closely packed within other
residues (166). One the other hand, Aro2 with mutations in F269 and F386 seemed to
have only a modest defect in binding and the least severe phenotype of the four mutants.
The complete loss of function of Aro1 is likely due to the greater disruption of the
structure of binding domains than Aro2. However, it should be noted that my single
molecule data showed that all three partially functional mutants, including Aro2, were
unable to form stable complexes with short ssDNA and have repair defects.
RPA-ssDNA interactions are the result of binding of multiple nonequivalent
domains. Each DBD in RPA individually has a low affinity for DNA and the affinity of
individual domains differ. However, DBD-A and DBD-B function together to form a
high affinity DNA binding site (32,161). The occupancy of DBD-C can increase the
affinity of RPA up to 20-50 fold. The crystal structural of Ustaligo Maydis RPA binding
to ssDNA also suggested that the centrally located DBD-B might coordinate DBD-C
binding. This effect could explain the more severe phenotype associated with AroB as
compared to AroA and Aro2. Since DBD-A and DBD-B are connected by a very short
linker, each could partially compensate for reduced binding of the other. However, the
polarity of RPA binding means that DBD-A positioned at the 5’ side of the complex and
DBD-B on the 3’ side of the complex closest to DBD-C. This means that reduced
binding of DBD-B (as in AroB) would be expected to have a bigger effect on the binding
of DBD-C, which is connected by a longer linker. This could explain the more severe
phenotype of AroB.
Regulation of RPA binding
RPA interacts with proteins involved in DNA replication, repair and
recombination and these interactions help regulate RPA function. Protein interactions
sites have been mapped to all the domains of RPA. DBD-A and DBD-B bind to a
118
number of DNA-processing factors, such as the replication protein Pol α primase, the HR
protein Rad52 and the NER factor XPA. However the interactions that have been mapped
precisely on DBD-A and DBD-B are outside the DNA binding sites.
My studies show that modest changes in the activity of one DBD can dramatically
affect function.
So RPA-protein interactions that modulate the binding of a single
domain (or alter the conformation of RPA so that domains have altered access to DNA)
will regulate function. Conversely, RPA-protein interactions are expected to be
modulated by changes in RPA conformation induced by binding or by transitions
between different binding modes (101).
An example of this is the RPA-mediated
polymerase switch during Okazaki fragment synthesis which is thought to be regulated
by a conformational change in RPA (130).
In response to DNA damage, RPA becomes hyper-phosphorylated. The hyperphosphorylation of RPA affects RPA binding and interactions with cellular proteins.
Current data indicates that hyper-phosphorylation causes a conformation change that
down-regulates activity in DNA replication but does not affect repair process (34,118). It
was also found that several residues in DBD-B, including W361, became surfaceinaccessible upon hyper-phosphorylation of RPA2 (118). Hyper-phosphorylated RPA
also has reduced affinity for the short ssDNA (118). This demonstrates that
posttranslational modification of RPA also regulates DNA binding and RPA function by
modulating the specific-domain interactions with ssDNA. This highlights the importance
of regulation of DBD-A and DBD-B in RPA function.
The conformation and dynamics of RPA-DNA complex
Structural analysis has suggested that RPA remodels its structure as it binds
ssDNA (28,46,49,52,156). By combining NMR, small angle scattering and
computational methods, it was possible to study the structures of intact human RPA as it
engages ssDNA. It has been shown that binding of ssDNA makes RPA undergo a two-
119
step compaction when transit from the low affinity binding mode to high affinity binding
mode (52). However, it is still challenging to make direct observations on domain
engagement and conformational changes when RPA binds DNA.
The use of single molecule TIRF microscope has allowed me to examine the
structure and dynamics of RPA-DNA complex. We immobilized the Cy5 labeled RPA on
a slide surface and allowed it to interact with Cy3 labeled DNA. By monitoring the
strength of FRET and changes of FRET, it provided us information on the domain
interactions and conformational change of RPA-DNA complex. These studies did not
identify large global conformational changes or diffusion of RPA along the ssDNA
during binding. However, we can’t exclude the possibility of RPA domains undergo
microscopic dissociation. We showed that internally labeled DNA bound by RPA
showed FRET changes on the 1-3 second time scale. This is likely a result of
microscopic dissociation and/or domain movements that bring two dyes together inside
the complex. However, movements of RPA domains might be very fast (<100 mS). If
so, we would be unable to detect such fast movements with labeled RPA under our
current experimental conditions.
Interestingly, my data suggests that DBD-F contributes to the dynamics of the
complex during some binding events. I propose that DBD-F occasionally interacts with
ends of DNA and brings the DNA close to the DBD-A. Structural studies indicate that
the long linker connecting DBD-F to DBD-A allows it to remain autonomous from the
DNA-binding domains that engage DNA in the 30 nt complex. DBD-F can interact with
both ssDNA and a number of proteins. It is the domain responsible for recruiting protein
partners into the RPA binding complex. This suggests that both protein binding and/or
DNA binding to DBD-F might be a driver of RPA remodeling.
My data shows that the primary determinant of FRET signal in RPA-DNA
complexes is the initial binding position of RPA along ssDNA. Also, the location of RPA
binding along the ssDNA is not correlated with length of binding events. This suggests
120
all the complexes examined are forming “stable”, 30 nt binding with engagements of four
DBDs. My observations do not have sufficient resolution to determine the order of
engagement of individual domains. However, recent studies (54,122) and my studies
suggest that RPA binding is not just the simple sequential engagement of DBDs proposed
previously. Among the four DBDs interacting with DNA, DBD-A binds to ssDNA with
greater affinity than the other DBDs, but because only a short linker separates DBD-A
and DBD-B, the local concentration of DBD-B is high when DBD-A binds to ssDNA.
The binding of two domains to ssDNA increases overall affinity of binding. DBD-C
binds DNA with weak affinity and the trimerization core of RPA, which is composed of
DBD-C, DBD-D and DBD-E, also can sufficiently bind to partial duplex with a 5’
ssDNA overhang of either 10 or 30 nt (27,138). It is likely that initial binding requires at
least two DBDs and that any initial association would increase the local concentration of
the other DBDs and facilitates the formation of a stable RPA-DNA (32,33). This model is
also consistent with my finding that there are at least two states of RPA-DNA complexes.
In single molecule analysis of RPA binding, we discovered that the dissociation
rate of RPA has both fast and slow phases. Such two-phase dissociation was not
identified previously in kinetic studies including previous stop-flow kinetic analysis
(54,167). While stop-flow analysis measures an entire ensemble of molecules, the single
molecule analysis allows us to extract detailed dynamical information from a large
number of individual binding events. This allows us to detect two-phase dissociation that
has been previously obscured in ensemble studies as a result of dephasing (148).
We also showed that mutations at the aromatic residues altered the two-phase
dissociation of RPA. The presence of slow-off rate phase is correlated with stability of
the RPA-DNA complexes that have long dwell times. We proposed that there are
multiple RPA-DNA complexes, either due to the different domains interacting with the
DNA or different conformations (Figure 4.1). Any disruptions of these interactions could
121
affect the stability of RPA complex and RPA function. We speculate that the ability for
RPA to form long binding events is important for RPA function in DNA repair.
Future directions for study of the aromatic mutants
In the future I would like to look further into the RPA-DNA interactions using the
single molecule methodology. So far, we have learned the general spatial location of
DBD-F and DBD-A in the RPA-DNA complex. However, the locations of the other
domains in the complex and the relative distances between different domains in the
complex remain to be determined. This will require additional forms of single and
double-labeled RPA. Such studies will allow us to build a more complete image of RPA
complexes with ssDNA. Future studies will provide a better understanding of molecular
interactions and RPA dynamics when binding to ssDNA and partially duplex structures.
I would also like to fluorescently label the Aro mutants and directly determine how these
mutations affect the RPA-DNA complex. With labeling the Aro mutants, we can
determine how the conformations and domain interactions in the Aro mutants differ from
those of wild-type RPA.
DBD-F can adopt many conformations. My study suggests that DBD-F plays a
role in regulating the dynamic RPA-DNA interactions. DBD-F exhibits weak affinity to
ssDNA and interacts with other proteins. It’s been proposed that DBD-F has a regulatory
role (30). This proposal was based on a study that showed that DBD-F interacts with the
phosphorylation domain of the RPA2 subunit and blocks undesirable interaction with the
core DNA-binding domain of RPA (30). It’s likely that through interacting with DBD-F,
proteins can modulate RPA binding and thus direct RPA to function in different
pathways. I have preliminary results that showed modifications on DBD-F altered RPA
binding in single molecule analysis. I would like to look into the role of DBD-F in RPA
binding and determine the interaction of DBD-F with DNA substrates by labeling DBDF.
122
Summary
RPA is essential for replication, repair and recombination. My studies illustrate
the different DNA metabolic pathways requires different RPA-DNA interactions. The
ability of RPA to form stable complexes with short ssDNA and melt secondary DNA
structure is needed for RPA function in repair. High affinity of RPA is not enough for
RPA to function in different pathways. My results suggest that RPA binding has at least
two states and that these probably involve both domain interactions and conformational
changes. The regulation of RPA dynamics when binding to ssDNA is likely to be
important for RPA to correctly process ssDNA intermediates in different pathways and
maintain genome integrity.
123
Figure 4.1. Possible mechanisms of RPA binding
124
APPENDIX I
FUNCTION OF RPA4 IN CELLULAR DNA DAMAGE REPAIR AND
PROLIFERATION
Abstract
Genome integrity and viability of cells are constantly threatened by DNA damage
from exogenous and endogenous reagents. High fidelity of replication and efficient DNA
repair are required to maintain genome integrity. Replication protein A (RPA) is essential
for DNA replication, repair and recombination. RPA is a heterotrimeric protein complex
composed of RPA1, RPA2 and RPA3. Normal human tissues also express a homolog of
the RPA2 subunit called RPA4. RPA4 can substitute for RPA2 to form an alternative
RPA complex (aRPA) complex. Current data indicates that aRPA functions in repair but
does not support replication. It is proposed that RPA4 can function in genome
maintenance in non-proliferating cells. My results confirmed that aRPA is unable to
support S phase progression and inhibits replication in proliferating cells. On the other
hand, initial studies suggest that RPA4 participates in NER repair and supports cell
recovery after DNA damage. In this chapter, I describe progress in developing methods
to look at the cellular functions of RPA4. These methods include a DNA damage
recovery assay, developing a Tet-off inducible system for expressing RPA4 in cultured
cells, and initial studies of immunostaining RPA4 in tissues.
Introduction
Single strand DNA-binding proteins (SSBs) that bind to ssDNA are essential for
DNA replication, recombination and DNA repair. SSBs have been identified in all
organisms including Escherichia coli, bacteriophages, yeast and humans (41). The human
SSB, known as RPA, is a trimeric complex that is composed of RPA1, RPA2 and RPA3
subunits. RPA homologues are found in all eukaryotic organisms including: other
mammalian species (168,169), Xenopus laevis (170), Drosophila melanogaster (19) and
125
unicellular organisms such as Saccharomyces cerevisiae (88,171) and Crithidia
fasciculate (172). Some organisms, such as seed plants (e.g. rice, Arabidopsis thaliana)
and some protists, contain multiple copies of one or more RPA subunits and can form
multiple RPA complexes (40,173). In the case of plants, the different RPA complexes
appear to have different functions (38). For example in rice, there are three different
genes encoding the largest (RPA70kDa) and middle subunits (RPA32kDa), but only one
gene encoding the smallest (RPA14kDa) (38). The various subunits do not randomly
associate with other subunits, but form distinct complexes. Three different RPA
complexes called type A, B and C RPA complex are composed of different combination
of subunits (38). Type A complex is localized in the chloroplast, but type B and type C
are found in the nuclear. It’s been suggested that type A RPA complex is required for
chloroplast DNA metabolism, whereas types B and C function in nuclear DNA
metabolism (40).
In human cells, a single homolog of the RPA2 subunit, called RPA4, has been
identified. RPA4 was isolated from a HeLa cell library interaction-trap/yeast two–hybrid
screen as a factor that interacts with RPA1 (41). RPA4 is intronless and resides on the Xchromosome at position q21.33. RPA4’s complete coding sequence lies in the intron of a
known coding gene, diaphanous2 (DIAPH2) (42). DIAPH2 encodes a formin-related
actin binding protein (174). Even though RPA4 expression is not well-understood,
available public data indicates that RPA4 is expressed in different levels and in different
tissues than DIAPH2 (42), suggesting that it is independently regulated. RPA4-related
sequences are only found in mammals and only primates and horse contain complete
coding sequences for RPA4 (42).
Sequence analysis revealed that RPA4 shares 47% amino acid sequence identity
and 63% amino acid similarity to RPA2 (42,175). RPA2 can be divided into three
domains: the N-terminal phosphorylation domain, the central DNA-binding domain D
(DBD-D) domain, and the C terminal region containing a linker and a winged helix
126
domain (WH). RPA4 has the similar domain organization to RPA2 (Figure. AI.1).
Nevertheless, the DNA-binding domain G (DBD-G) of RPA4 is functionally different
from DBD-D domain of RPA2 (176). While DBD-D domain of RPA2 is essential for
cellular replication, DBD-G domain of RPA4 is defective for replication and causes
unstressed cells to arrest in G2/M (176).
RPA4 can substitute for RPA2 to form an alternative RPA complex (aRPA) that
has biochemical properties similar to canonical RPA (177). In contrast to the canonical
RPA, which supports DNA synthesis in the SV40 replication system in vitro, aRPA
failed to substitute for RPA and inhibited DNA replication in the presence of canonical
RPA (43). This suggests that if both RPA4 and RPA2 are present in cells, RPA4 should
compete with RPA2 and inhibit DNA replication and cell-cycle progression. However,
one previous study showed no G2/M cell cycle arrest for cells expressing both RPA2 and
RPA4 (176). One explanation of these data was that RPA4 levels might not have been
high enough to observe a phenotype in the presence of RPA2. In the same study coexpression of a RPA2/4 hybrid (called RPA2 basic) with endogenous RPA2 caused cell
cycle arrest. RPA2 basic is a form of RPA2 when the acidic L34 loop in DBD-D from
RPA2 is replaced with basic L34 loop in DBD-G from RPA4 (176). This suggests RPA2
basic competed more efficiently with wild-type RPA2 than RPA4 and that the
competition between RPA2 and RPA4 in cells may be complex. Unpublished data from
the Wold lab also suggested that the expression level of exogenous RPA4 in transformed
cells is down regulated. This suggests that RPA2 Basic and probably RPA4 can inhibit
normal RPA2 functions in cells when expressed at high enough levels. However, the
normal expression level of RPA4 in different types of cells remains unknown.
Biochemical experiments suggested that the replication defect in aRPA was
caused by an inability to support efficient pol α loading (177). In contrast to being
nonfunctional in replication, aRPA is able to support nucleotide excision repair, Rad51dependent DNA strand exchange and checkpoint activation (44,178). Nucleotide excision
127
repair (NER) is the DNA repair pathway for the removal of helix-distorting lesions from
DNA induced by agents like UV light from the sun (179). RPA participates in multiple
steps in NER(180-182), including binding to XPA to aid in cooperative recognition of
DNA damage and stimulation of XPF-ERCC1 nuclease activity (183,184). By using an in
vitro reconstituted reaction, aRPA was shown to support the dual incision/excision
reaction of NER (44). However, aRPA seemed to be less efficient in NER than the
canonical RPA having reduced interactions with the XPA and not stimulating XPFERCC1 endonuclease activity (184).
Another DNA repair pathway is homologous recombination (HR), which allows
cells to repair double-stranded DNA breaks (DSBs) (173). Both in human and yeast, HRmediated repair requires a homology search and DNA invasion by the Rad51-ssDNA
filament, positioning the invading 3’ end on a template duplex DNA to initiate repair
synthesis (185). RPA interacts with both Rad51 and Rad52 (66,186,187). Rad51 filament
formation is greatly simulated in the presence of RPA (188). In yeast, Rad52 modulates
filament formation by promoting strand annealing by efficiently removes RPA from
ssDNA in HR repair (189,190). It has been shown that aRPA is also able to interact with
both Rad51 and Rad52 and stimulated Rad51 strand exchange (44). Nevertheless,
whether RPA4 can efficiently substitute for RPA2 in repair of different types of DNA
damage in cells needs to be determined.
Quantification of RPA4 mRNA level in different human tissues has shown that
RPA4 is expressed in 20 normal tissues (44). RPA4 mRNA level was detected at levels
similar to or above RPA2 mRNA in some tissues including colon, bladder, esophagus,
lung, and prostate (43). The reasons for different RPA4 levels in different tissues are not
known. In cancerous tissue, RPA4 mRNA was expressed at reduced levels (43). The
level of RPA has been shown to be a prognostic indicator in colon cancer patients (191).
It was reported that there were gradual increases in expression of RPA1 and RPA2 as
tissues evolved from normal to cancerous tissue (191). It is interesting to speculate that
128
this increase is concurrent with the observed decrease in RPA4 expression upon
transformation. Currently it is not known which cell types express RPA4 in individual
tissues or what the RPA4 protein levels change during normal cellular processes.
The goal of my studies was to determine the cellular functions of RPA4. I used a
knockdown and reconstitution system in cultured cells to explore the roles of RPA4 and
aRPA in DNA replication and repair. The ability of aRPA to function in replication was
determined by whether cells expressing exogenous RPA4 could progress through S phase.
I also examined the localization of exogenous RPA4 to DNA damage sites and repair
functions. I especially focused on the role of RPA4 in nucleotide excision repair (NER).
During NER, proteins involved in repair become tightly associated with chromatin. So I
also established methods to examine chromatin bound proteins after DNA damage or UV
irradiation. These studies were technically challenging because I found that expression of
RPA4 prevents cell proliferation. Therefore, I developed and carried out initial
characterization of stable cell lines that have inducible RPA4 expression using lentiviral
Tet-off promoters. Finally, I carried out immunostaining on human colon cryosections to
try to determine the distribution of RPA4 expression in a normal tissue. These studies
laid the foundation of future studies on role of aRPA in cell proliferation and repair.
Materials and Methods
RNAi knockdown and replacement of RPA2
Methods for knock down endogenous RPA2 and expression of exogenous RPA2
and RPA4 were as described as previously (31). Briefly, HeLa cells (obtained from the
American Culture Collection) grown in DMEM with 10% calf serum at 37 °C with 5%
CO2 are seeded in six-well tissue culture plates with 2x105 cells per well. Small
interfering siRNA (siRNA) (200 pmol) was transfected 24 hours after seeding to
knockdown the endogenous RPA2. Transfections were done with 5 µl of lipofectamine
2000 (Invitrogem). 24 hours after transfection of siRNA, cells were transfected with 250
129
ng of plasmid expressing GFP fusion of RPA2 or RPA4 (42). The RPA2 siRNA target
sequence was 5’-CCUAGUUUCACAAUCUGUUUU- 3’(192).
Flow Cytometry analysis
Methods for doing flow cytometry were as described (143). Cells were collected
after 96 hours posttransfection of siRNA, washed with PBS, and fixed overnight in 70%
methanol. The cells were rehydrated in PBS for 30 minutes and washed in PBS. For cell
cycle analysis, 0.1% mg/mL propidium iodide was added to each sample. Cells were
examined on a FACScan II, and the data were analyzed using the FlowJo software
(TreeStar). For synchronization studies, at 72 hours after siRNA transfection, cells were
treated with 5 µg/ mL aphidicolin for 24 hours. The cells were released into fresh
medium and collected at 0, 8, and 24 hours after release.
Immunofluoresence analysis and DNA damage assays
Methods for immuofluoresence immunostaining were as described (193). HeLa
cells were seeded on coverslips in six-well tissue culture plates, and subjected to RNAi
knockdown and replacement of RPA2 and RPA4 as described (193). At 92 hours posttransfection of siRNA, 20 µmol/L camptothecin was added to each well. The cells were
incubated for 4 hours at 37 °C with 5% CO2. Coverslips were washed twice in cold CSK
buffer (10 mmol/L HEPES, 300 mmol/L sucrose, 100 mmol/L NaCl, and 3 mmol/L
MgCl2). Non chromatin-bound RPA was extracted with CSK/ 0.5% Triton X-100 for 5
minutes. Coverslips were fixed with 4% formaldehyde for 20 minutes then washed twice
with PBS. To detect phosphorylated H2AX (p-H2AX), coverslips were incubated in
blocking solution (5% calf serum, PBS) for 1 hour at room temperature then in primary
antibody at 1:500 overnight at 4°C. Primary antibody used for phosphorylated H2AX (pH2AX 1:600) (Cell Signaling). Coverslips were washed three times with PBS and then
incubated with anti-rabbit Texas red secondary antibody (Cell Signaling) at 1:800 for two
hours. Coverslips were washed in PBS, incubated in DNA staining solution DAPI (1µg/
130
L) for 5 minutes, washed in PBS and mounted on a slide. Slides were examined with a
Leica immunofluorescence microscope and images were collected with SPOT software
(Diagnostic instruments, Inc). Confocal images of replication foci were collected with a
Zeiss 710 Confocal microscope. ImageJ and Adobe photoshop were used to process and
overlay images.
For the 4NQO pulse and recovery assays, cells were grown on coverslips as
previously described. At 93 hours post-transfection of siRNA, cells were exposed to 10
µM or indicated concentration of 4NQO. After 3 hours, cells were released into media
and allowed to recover and were collected at 0 and 24 hours after release. Cells were
stained with antibodies as described above and percent of cells with activation of DNA
damage markers were quantified visually.
Cell UV irradiation
Cells were cultured and grown as described above. After 96 hours posttransfection of siRNA, cells were subjected to UV irradiation. The growing medium was
removed and cells washed twice with PBS. Plate was uncovered and placed on the
surface of the tissue culture hood under the UV lamp. The UV lamp was adjusted so that
the surface was receiving 50 µW/ cm2 (UVP UVX Digital Ultraviolet Intensity Meter)
(Cole-Parmer). At this setting, cells were irradiated at a dosage of 0.5 J/m2/s. After UV
irradiation, media was added and the cells grown as described above until harvest and
processing.
Chromatin-bound fractionation and immmunoblotting
Methods for cellular fractionation were done as described(194). Cells were
harvested from six-well plate using trypsin. Cells were centrifuged at 1000 rpm for 5
minutes and the supernatant removed. Cells were washed with PBS and centrifuged at
1000 rpm and the supernatant discarded. Cells were re-suspended in 200 µl of solution A
(1 mM HEPEs, pH7.9, 10 mM KCl, 1.5 mM MgCl2, 0.34 M sucrose, 10% glycerol,
131
0.1%Triton-100X, 10 mM NaF, 1mM DTT, 1 mM sodium vandate, 1mM PMSF and
protease inhibitors (complete protease inhibitor cocktail tablets (Roche)) and incubated
on ice for 10 minutes. After vortexing vigorously for 10 sec, cytoplasmic proteins were
separated from nuclei by low-speed centrifugation 5000 rpm for 5 minutes at 4°C.
Supernatants were removed and isolated nuclei pellets were re-suspended in 100 µl of
solution A. Nuclei were left in ice for 10 minutes. The nuclei were centrifuged at 5000
rpm for 4 minutes at 4°C and the supernatant removed. Nuclei were re-suspended in 200
µl of solution B (3 mM EDTA, 0.2 mM EGTA, and 1 mM DTT and protease inhibitors).
After 30 minutes incubation on ice, supernatant was separated from chromatin by
centrifugation on 6500 rpm for 4 minutes at 4°C. Isolated chromatin was washed once
with solution B and centrifuged at high speed at 14000 rpm for 1 minute at 4 °C. Finally,
chromatin was re-suspended in 100 µl of loading buffer (Laemmli buffer) and sheared by
sonication. Equal loading of chromatin fractions from samples were verified by
immunoblotting against Histone H3 (Cell Signaling Technology). Primary antibody used
is XPA (FL-273) at 1:500 (Santa Cruz biotechnology).
Colon tissue immunohistochemistry
The frozen colon tissue sections were warmed to room temperature and then
immersed in pre-cooled acetone (-20 °C) for 10 minutes in a glass slide-staining jar.
Acetone is removed and the slides incubated for more than 20 minutes at room
temperature to allowed the acetone to evaporate. Slides were rinsed three times with
CSK buffer, 5 minutes each. The tissue slides were fixed with 4% formaldehyde in PBS
for 15 minutes. Then, slides were permeablized with 0.5% tween in PBS for 5 minutes.
Slides were then rinsed in PBS three times, 5 minutes each. 2-3 drops of Background
Buster (INNOVEX Biosciences) are added to the slide surface for blocking and incubated
for 30 minutes in a humid environment at room temperature. The slides were then
washed with PBS three times. Slides were incubated with primary antibody diluted in
132
blocking buffer (5% donkey serum, 0.1% tween-20 in PBS) overnight at 4°C. The
primary antibodies used were mouse anti-RPA1 (2H10B -1: 2500) and sheep anti-RPA4
(1:2500). Slides were washed three times with PBS and incubated with goat anti-mouse
Alexa Fluor 488 (1:3000) and anti-sheep Texas red (1:3000) secondary antibody in 0.1%
tween-20 PBS for 2 hours in room temperature. Slides were washed three times with PBS
and incubated in DNA staining solution DAPI (1 µg/L) for 5 minutes, then washed and
mounted. Slides were examined with a Leica immunofluorescence microscope and
images were collected with SPOT software (Diagnostic instruments, Inc). ImageJ and
Adobe photoshop were used to process and overlay images.
Lentiviral inducible Tet-off RPA expression constructs
Lentiviral pFIV3.2TRE plasmid was acquired from Gene vector core. To make
pFIV3.2TRE puro, the SV40 promoter and puromycine CDS fragment from pBABEpuro-mcse plasmid were amplified by PCR using primer: SV40 promoter F: 5’CCCAGCAGGCAGAAGTAT-3’, SV40 promoter R: 5’TAGCTTGCCAAACCTACAGGTGG-3’. The PCR product was cloned into the pCR2.1
TOPO vector to make the pCR2.1 TOPO puro. Then SV40 promoter and puromycin
CDS from pCR2.1 TOPO is ligated to pFIV3.2 TRE using the EcoRI and ClaI sites. To
make pFIV3.2TRE puro RPA2 and RPA4, the GFP-RPA2 and GFP-RPA4 coding
sequences were amplified from pEGFP-RPA2 or pEGFP-RPA4 vector. HpaI and PacI
sites were integrated into the primers for PCR: pEGFPRPA4HpaI F: 5’GTTAACACGAACCGTCAGATCCGCT-3’, pEGFPRPA4PacI R: 5’TTAATTAAGATCCGGTGGATCCCG-3’. The amplified PCR fragments were cloned
into the pCR2.1 TOPO to make pCR2.1 TOPO RPA2 and RPA4. GFP-RPA2 and GFPRPA4 are ligated to pFIV3.2TRE puro using HpaI and PacI site. The resulting plasmids
were given to the Gene Vector Core who used them to make Lentivirus.
133
Tet-off inducible system
The stable Tet-off HeLa cell line (Clonetech) that expressed the advanced
transactivator was used. The HeLa Tet-off cell line was selected with G418 and is
maintained with G418. Tet-off HeLa cells were seeded in in six-well tissue culture plates
with 2x105 cells per well and grown in DMEM with 10% FBS serum (Tetracycline-free)
(Clontech) with 1µg/ mL Doxcycline (Clontech) and 100 µg/mL G418 at 37 °C with 5%
CO2. The 250 ng of lentivrial vector pFIV3.2TRE puro GFP-RPA2 and GFP-RPA4 or the
lentivirus carrying the lentiviral vector (vector core) were introduced into cells 48 hours
after seeding. Plasmid transfections were done with 5 µl of lipofectamine 2000
(Invitrogen). Virus infections were done with 1mL of 2% FBS contains 1 MOI (40000
TU/Cell) of virus and polybrene (4µg/mL) (Santa Cruz Biotech). The 2% FBS was
removed 4-8 hours after infection and replaced with complete media. The media was
now switched back to 10 % FBS (Tetracycline-free) without adding Doxycline in order to
see induction of RPA2 and RPA4. Cells were collected at 72 hours post-transfection of
plasmid or virus and analyzed for GFP expression by flow cytometry as described above.
Making Tet-off cell line and double-stable Tet-off inducible cell
line
The methods for making Tet-off and double-stable Tet-off inducible cell line were
as described in the Clontech Tet-off advanced inducible gene expression systems user
manual. The Tet-off cell lines can also be made in other cell line such as U2OS and
HeK293T cells by transfecting cells with pTET-Off vector (Clonetech) and using the
antibiotic G418 to select for stable cell lines. To make the double-stable Tet-off inducible
HeLa cells, the lentivirus carrying the pFIV3.2 TRE puro GFP-RPA2 and GFP-RPA4
were infected to HeLa Tet-off. Briefly, HeLa Tet-off cells were grown in DMEM with
10% FBS serum (Tetracycline-free, Clontech) with adding the 1µg/ mL Doxcycline
(Clontech) at 37 °C with 5% CO2 in six-well tissue culture plates seeded with 2x105 cells
134
per well. Virus infections were done with 1mL of 2% FBS containing 1 MOI (40000
TU/Cell) of virus and polybrene (4µg/mL) 48 hours after seeding. The 2% FBS was
removed 4-8 hours after infection and replaced with complete media containing 1µg/ mL
Doxcycline. 24 hours after infection, the infected cells were transferred to 10 cm plates
and grown in media containing 100 µg/mL G418 and 1 µg/mL of Dox for 48 hours
before adding the puromycin (1 µg/ mL). The drug selection continued until colonies
become visible which usually take 2-4 weeks. Colonies were picked using cloning
cylinders (Corning) and transferred to six-well tissue culture plates. Cells were cultured
in medium containing 100 µg/mL G418, 1 µg/mL puromycine and 1 µg/mL of Dox. Cells
from each colony were grown in Dox-free medium and screened for GFP expression
induction using flow cytometry.
Affinity purification of RPA4 antibody
Sheep polyclonal antiserum made against RPA4/3 complex was affinity purified.
1 ml of Actigel ALD (Sterogene) beads was exchanged into PBS and incubated with 1
mg of His-tagged RPA3/4 complex protein in ALD coupling solution (Sterogene) at
room temperature for 2 hours. The beads and proteins are transferred to 10 mL disposable
column. The column was washed with 10-column volumes of PBS until the OD reading
is less than 0.1. The filtered 5 mL RPA4 anti-serum was loaded to the column, and flow
through collected. The flow through was then passed through the columns six additional
times. The column was then washed with 10-column volume of PBS until OD reading
was less than 0.1. Antibody was eluted with 100 mM Glycine pH 2.5 in 500-µl fractions
to tubes that contain 75 µl of 0.3 M Tris pH 10.4 and 0.7 M KCl.
135
Results
RPA4 is unable to substitute for RPA2 to rescue cell cycle
progression
I initially wanted to determine whether RPA4 could substitute for RPA2 in human
cells. Our lab has previously developed a knockdown reconstitution system, in which we
knockdown endogenous RPA with siRNA and substitute with exogenous gene expression
to study the function of RPA variants in cultured cells. HeLa cells were treated with a
siRNA to target to the 3’ untranslated region of RPA2. This reduced endogenous RPA2
protein levels to less than 5% of normal levels (31,42). In this system, the maximal
knockdown of RPA2 occurred between 72-96 hours after siRNA transfection and
resulted in coordinate depletion of RPA1 protein (42). 24 hours after transfection of
siRNA, a plasmid expressing GFP-RPA2 lacking the targeted 3’ untranslated region or
GFP-RPA4 were introduced to the RPA2 depleted cells, and the distribution of cells in
the cell cycle was determined by flow cytometry analysis after propidium iodide staining
of DNA. Both plasmids contain a N-terminal GPF tag (31). As has been observed
previously, RPA2 depletion caused an accumulation of cells in S phase and G2/M phase,
indicating replication and repair defects (189). The cells expressing exogenous RPA2 and
RPA4 were identified as GFP-expressing cells by flow cytometry and were analyzed for
cell cycle distribution. Cells expressing GFP-RPA2 had a regular cell cycle distribution
similar to mock-transfected cells (Figure. AI.7 B) (42). This confirmed that GFP-RPA2
was functional in cells. Cells expressing exogenous RPA4 have a fewer cells in G2/M
phase than RPA2-depleted cells, but an increased percentage of cells in S-phase cells
compared to cells rescued by RPA2. In vitro studies on aRPA function in SV40
replication previously showed that aRPA is unable to support replication (43). Thus, the
increased S-phase in RPA4-expressing cells is consistent with the conclusion that RPA4
has defective in DNA replication.
136
To confirm these findings, I examined the ability of RPA4 to support DNA
replication in synchronized cells. After knockdown and reconstitution, cells were treated
with the DNA polymerase inhibitor aphidicolin (APH) for 24 hours to synchronize the
cells at the G1/S boundary. Cells were released in APH-free media, and S-phase
progression was monitored after 0, 8 and 24 hours. The mock-treated cells were the
positive control. At 0 hours, a majority of population of cells in all samples were in G1
phase (Figure.AI.2). After 8 hours, the mock-treated cells had cells entering S-phase as
indicated by thickening and shift of the G1 peak to the right (Figure.AI.2). Exogenous
RPA2-expressing cells also progressed through S-Phase as the 8 hour time point after
APH release (Figure.AI.2) indicating that replication was occurring in these reconstituted
cells. By 24 hours, exogenous RPA2-expressing cells had populations of cells either in
G2/M phase or re-entering G1-phase, similar to mock-treated cells (Figure.AI.2). In
contrast, exogenous RPA4-expressing cells were unable to progress-through S-phase 8 or
even 24 hours after APH release (Figure.AI.2). This result was consistent with aRPA not
supporting SV40 DNA replication in vitro and demonstrates that RPA4 is unable to
support replication in proliferating cells.
RPA4 can function in NER
RPA is required for repair of different types of DNA damage, including bulky
adducts, DSBs, and replication stress. In vitro studies have suggested that aRPA is able
to substitute for RPA in reactions in NER and DSB repair. It has also been shown that
RPA4 localizes to the repair foci in damaged cells after Camptothecin (CPT) treatment
(42). CPT causes single- and double-stranded DNA breaks (DSBs) (42). To determine
whether RPA4 is also able to localize to sites of NER repair, we utilized 4NQO, an
alkylating agent that causes modification of bases that are repaired by NER. Cells
expressing endogenous RPA2 or RPA2-depleted cells expressing GFP-RPA2 or RPA4
were treated with 4NQO (10 µM) for three hours. The level of DNA damage was then
137
monitored by following phosphorylated H2AX (γH2AX). H2AX is a variant of the H2A
protein family, which is a component of the histone octomer in nucleosomes (195).
H2AX is quickly phosphorylated upon double-stranded breaks (DSBs) induced by
ionizing radiation or DNA damage agents (196). Because phosphorylation of H2AX at
Ser139 (γH2AX) is abundant, fast and correlates well with damage, it is a sensitive
marker that can be used to examine the presence of DNA damage and subsequent repair
of the DNA lesions (196). In the presence of DNA damage, cell nuclei become positive
for γH2AX staining (Figure.AI.3). After addition of 4NQO, cells reconstituted with GFPRPA2 and GFP-RPA4 exhibited punctate staining pattern of RPA throughout the nucleus
in damaged cells. This result showed that GFP-RPA4 is able to localize to sites of DNA
damage repaired by NER, hinting RPA4 in participating in NER repair.
Because RPA4 can localize to the site of DNA damage repaired by NER, I went
ahead to try to determine whether cells expressing RPA4 could recover from 4NQO
damage. In these recovery assays, cells were treated with low dosage of 4NQO (0.05 µM)
for 3 hours, then fresh media was added and the cells grown for 24 hour. Endogenous
RPA2 was depleted and rescued with either GFP-RPA2 or GFP-RPA4 and recovery
monitored. I only quantitated the level of γH2AX in the GFP expressing cells to ensure
that only cells expressing the indicated form of RPA were counted. The γH2AX signal
intensity of individual nuclei was quantified using ImageJ and plotted as a dot plot. For
all 4NQO-treated cells, there was a significant increase in γH2AX signal intensity (p
<0.001). A decrease in the γH2AX signal intensity after 24 hours indicated that mock
and RPA2-expressing cells had recovered from 4NQO damage. No recovery was
observed in RPA2-depleted cells. RPA4-expressing cells showed partially recovery
indicating that at least some NER repair was occurring (Figure.AI.4). Previous studies
have shown that γH2AX was observed in undamaged cells expressing RPA4. This
suggests that RPA2-depeleted cells expressing RPA4 contain abnormal DNA structures
(42). We have previously showed that RPA4 does not support replication, thus cells
138
expressing RPA4 might lead to replication stress and generate abnormal DNA structures.
I conclude that RPA4 is able to participate in NER but RPA4 expression also caused
additional damage.
The NER specific-damage marker XPA is localized to
chromatin in response to 4NQO treatment
Because γH2AX is a general response to DNA damage, I also attempted to follow
XPA as a NER-specific damage marker. XPA is known to localize to damage sites during
NER independently of RPA, but establishment of the NER repair complex requires XPA,
XPC and RPA (197). XPA recognizes various NER-specific types of damage including
pyrimidine-(6-4)-pyrimidone photoproducts (6-4PPs) and cyclobutane pyrimidine
dimers(CPDs) and is needed to recruit core NER repair factors (179). The same recovery
assay was repeated by looking at the signal intensity of XPA. However, a high
background of XPA staining was observed. Currently, the low level of damage coupled
with the high XPA background has prevented me from making conclusions from these
experiments (Figure.AI.5). One possible complication is that it has been reported that
XPA nuclear import is cell cycle dependent and happens primarily in the S-phase (197).
This could also prevent me from observing a significant change in XPA nuclear intensity
since the duration of damage is short and a majority of HeLa cells in asynchronous
culture are in G1-phase.
As an alternative approach, I examined the chromatin-bound protein fraction
before and after damage to determine whether I could visualize changes in XPA
localization upon 4NQO treatment. Histone H3 was used as loading control for
chromatin-bound proteins. Cells were treated with 10 µM 4NQO for three hours, and the
chromatin fraction of XPA was isolated and detected by western blot. My preliminary
western blot data (Figure.AI.6 A) suggested that knockdown of RPA2 didn’t increase the
localization of XPA in the absence of damage. When cells were exposed to 4NQO, mock
139
and RPA2-recued cells show increased chromatin-bound XPA and RPA4-rescued cells
have even higher XPA localization. In the absence of damage, RPA4-rescued cells also
had more XPA localization than mock cells, which suggested that RPA4 expressing cells
might contain DNA damage that activates NER repair (or at least causes nuclear
localization of XPA). Repeated experiments and quantification is needed to confirm with
this result.
UV irradiation is used as an alternative way to induce NER
It was difficult to determine the optimal amount of 4NQO appropriate for damage
recovery assays. Also, 4NQO can’t be completely removed from cells after replacing
with fresh media, which could change the time course and interfere with efficiently
recovery. One alternative way to induce NER in cells is to use UV irradiation. It has been
shown that 10J/m2 UV could make XPA localize to nucleus in Hela cells, and cells
recovered efficiently from 10J/m2 UV (197). To determine the optimal UV intensity for
recovery in my assays, I did a titration of UV intensity. Cells were UV irradiated for
different lengths of time to achieve 5, 10, 15 J/m2 UV dosage. To compare UV irradiation
with 4NQO, cells were treated with 1 µM and 0.1 µM of 4NQO (Figure.AI.6 B). The
level of chromatin bound XPA was compared before and after DNA damage for both
DNA reagents. While UV irradiation induced strong localization of XPA, 4NQO-treated
cells exhibited low levels of XPA chromatin localization compared to the non-damage
cells. Also, cells recovered efficiently from UV irradiation. This result suggested that UV
irradiation is a better damaging agent for NER in these assays. Because 5 J/m2 UV
induced similar level of XPA localization as 15 J/m2, we decided to use 5 J/m2 to test
recovery.
The NER recovery assay was repeated for UV irradiated cells. The recovery of
UV irradiated cells reconstituted with GFP-RPA2 or -RPA4 was examined (Figure.AI.6
C). Mock treated cells and RPA2 knockdown cells were the positive and negative
140
controls, respectively. The preliminary results showed that both RPA2 and RPA4
reconstituted cells can recover from UV damage. To our surprise, RPA2 knockdown cells
also recovered from UV damage 24 hours after treatment. This is surprising because RPA
is essential for NER repair, and in the absence of RPA, we expected the NER repair
would be incomplete. Under these conditions accumulated NER intermediates should
lead to increased XPA localization. It is known that RPA and XPA act cooperatively to
recognize DNA lesion, but that RPA is not required for XPA recruitment to the damage
site(162,183). Since RPA2-depleted cells were also able to recover from UV irradiation,
we could not conclude that RPA4-rescued cells support NER repair from this study.
However, based on the previous experiments, cells expressing exogenous RPA4 are able
to partially recovery from NER. Additional studies are required to confirm this tentative
conclusion.
Another method that could be used to examine NER repair is immunostaining of
chromatin-bound proteins at repair foci. This method has a high signal to noise ratio
compared to standard immunostaining. However, the knockdown and reconstitution
system used in these studies only results in 20-30% of cells expressing exogenous
protein. This means that it is difficult to interpret experiments because more than half of
cells don’t express exogenous RPA2 or RPA4. We need to increase the transfection
efficiency and/or make stable cell line with inducible RPA4 expression in order to pursue
these studies further.
Developing double-stable Tet-off inducible cell line with
inducible RPA4 expression
We have previously used a constitutive FIV virus to express RPA4 in HeLa cells.
In these viruses, CMV promoter is used to drive RPA4 expression. Cells with RPA4
expression showed low viability in long-term growth compared to cells expressing RPA2
141
and RPA4 (Cathy Hass, personal communication). This also provides evidence that
RPA4 is not compatible with proliferation.
To develop a tractable system for studying RPA4 functions in vivo, I decided to
make an inducible, stable cell line that expresses RPA4. These cells will have inducible
RPA4 expression. Such cells have the potential to overcome the problems described
above with low transfection efficiency and toxicity associated with transient transfection
of plasmids directing RPA4 expression. The Tet-off inducible gene expression system
was utilized. In this system, the cells that contain an integrated TRE (Tetracycline
response element)-based expression vector and express the Tet-off advanced
transactivator can be induced to express a gene of interest when cultured in the absence
of doxycycline (DOX). In addition, the lentiviral gene delivery system should provide
efficient delivery and stable integration of the gene construct to cells. I acquired the
pFIV3.2TREmcs lentiviral vector from viral vector core. This plasmid vector contains a
TRE promoter. I then cloned GFP-RPA2 or GFP-RPA4 downstream of TRE promoter.
These plasmids also contained a separate SV40 promoter downstream, which directs the
expression of the Pac gene, which encodes a puromycin N-acetyl-transferase that causes
resistance to puromycin. The SV40 promoter drives the expression of Pac gene
independently from TRE-regulated promoter. The resulting plasmids, FIV3.2TRE puro
GFP-RPA2 and puro GFP-RPA4 had an inducible gene of interest and a constitutively
expressed selectable marker.
The commercially available HeLa Tet-off cells that stably express the Tet-off
transactivator were used for induction. I initially carried out proof-of-concept studies by
transient transfecting Hela Tet-off cells with the inducible lentiviral plasmids,
FIV3.2TRE puro GFP-RPA2 and puro GFP-RPA4. Transfected cells showed inducible
GFP expression in the absence of Doxycycline (Dox) (after growth in Dox free medium
for 48 hours) and no expression in the presence of Dox (Figure.AI.7 A). The exogenously
expressed GFP-RPA2 and RPA4 were functional in RPA2-depleted cells; the cycle
142
distribution observed was similar to that in previous RPA2- or RPA4-reconstituted cells
(Figure.AI.7 B). After showing that the constructed inducible plasmids are functional, we
sent the plasmids to vector core to make lentiviral virus.
Transduction of HeLa Tet-off cells with inducible
Lentiviral virus showed Dox-regulated GFP-RPA2 and
GFP-RPA4 expression
To characterize the inducible Lentivrial virus, virus transduction was carried out
in HeLa Tet-off cells and expression levels monitored in the presence and absence of
Dox. In the absence of Dox, the percentage of cells expressing GFP in infected cells was
around 20% to 30% at 48-72 hours post infection (Figure.AI.8 A). At similar times, I
observed suppressed expression of GFP in the presence of Dox (Figure.AI.8 A).
To make stable cells, I grew infected cells in media with both puromycin (1
µg/mL) and Dox (1µg/mL). Dox was always added to the media, because expression of
RPA4 was expected to lead to negative selection. After one week of selection, growing
cells were collected to makes an initial pool of puro-resistant cells. I kept half of cells
growing in plus Dox media and transferred the other half to minus Dox media (both
media also contained puromycin). The number of GFP expressing cells was determined
after four days in Dox free media using flow cytometry (Figure.AI.8 B). I observed 2 %
GFP positive cells for the RPA2 infection and 1% for RPA4 infection. In order to
determine how long it took to get the highest induction, the same pools were grown for
several weeks in minus Dox media. The highest induction obtained in any experiment
was 6% for RPA2 and 2% for RPA4 infected cells and this occurred in cells that had
been grown in minus Dox media for 17 days (Figure.AI.8 B). This indicated that after
puromycin selection, a majority of cells have lost inductivity.
143
Selected colonies showed low inductivity and cells with
inducible RPA4 expression are negatively selected
To obtain a homologous population of cells, I picked single colonies from puroresistant cells. I started by collecting 30 colonies from each RPA2 and RPA4 transduced
cells. All colonies were then examined after growth for five days in minus Dox media.
After this induction, 1 to 2% of cells in all of the colonies had induced GFP expression.
To explore the stability of these cells, one colony from each RPA2 and RPA4
transduction was selected for long-term growth. The colonies were kept growing in up to
28 days in Dox free media. The highest induction was observed at day 12 and day 14 for
RPA2 and RPA4 transduced colonies, which had 10 to 12% of cells showing GFP
expression (Figure.AI.9). A gradual decrease in induction of GFP expression was
observed in RPA4 transduced cells, which had 4% of GFP expressing cells by day 28. On
the other hand, the induction of GFP-RPA2 was maintained at 12% by day 26. This
preliminary result suggested that RPA4 expression incompatible with proliferation, and
RPA4 expressing cells were negatively selected. So far, all colonies showed delayed
induction and also low inductivity. Because less than 6% of puro-resistant cells had
induced GFP expression when collected as a pool (Figure AI.8 B), I speculated that I
didn’t obtain large enough number of colonies to obtain inducible cells.
Determine the distribution of RPA4 in normal and
transformed tissues
RPA4 mRNA has been detected in all normal human tissues. However, the
expression level and distribution of RPA4 protein in different cell types is still unknown.
We decided to look at the distribution of RPA4 in normal human tissue by
immunostaining. We hypothesized that RPA4 expression would be elevated in
differentiated cells and suppressed in proliferating cells. Colon tissue was used because it
has high levels of RPA4 mRNA, a defined morphology and contains both differentiated
144
and proliferating cells (188,198,199). Colon tissue consists of millions of crypts. The
epithelial layer of the crypt is made up of a single sheet of columnar epithelial cells,
which are surround by underlying connective tissue of the lamina propria (198). The
epithelial cells turn over quickly and constantly; all cells except for stem cells are
replaced within seven days (199). Studies have suggested that the colon crypt is
maintained by stem-cell population at the base the crypt, within the stem-cells niche,
formed by stem cells and mesenchymal cells that surround the crypt base (199). Stem
cells divide into more differentiated cells which migrate to the surface of colon and
subsequently die (199). Immunohistochemical analysis in control normal human colon
has revealed the presence of RPA1 and RPA2 in colonical epithelial cells in the lower
two-thirds of the crypts (199). Because RPA4 cannot support replication, we expect to
see a differential expression of RPA4 in epithelial cells, in which the upper parts of crypt
would have enhanced RPA4 expression compared to the cells in the proliferative
compartment.
I decided to look at RPA4 expression in colon crypt cryosections by
immunostaining. Our lab has previously generated anti-RPA4 serum. Preliminary studies
on colon immunstaining showed high background for RPA4 detection. As the positive
control, anti-RPA1 antibody was used. RPA1 staining overlaps with DAPI staining in
crypt, indicating the nuclear localization (Figure.AI.10). Compared to RPA1, there was
no distinctive nuclear localization for RPA4 and non-specific binding to surrounding
tissue was observed. This could either be caused by low expression of RPA4 in epithelial
cells or non-specific binding of antibody. To rule out the latter reason, I affinity purified
the anti-RPA4 serum to improve the specificity of the RPA4 antibody. I then
characterized the affinity purified-RPA4 antibody using Western blotting. The result
showed that purified-RPA4 antibody has reduced cross reactivity with the RPA3 subunit
as compared to the RPA4 antiserum (Figure.AI.11). Further studies are needed to
optimize immunostaining conditions using the affinity purified-RPA4 antibody in HeLa
145
cells that are transiently expressing RPA4 and then examine RPA4 expression in colon
crypt cryosections. We expect to see improved signal to background ratio with the
purified-RPA4 antibody.
aRPA showed altered interaction with slipped-DNA
structure within the CTG/CAG repeat
Recently, it was found that RPA4 mRNA level is greatly increased in the brains
of Huntington’s disease (HD) patients, while the expression of RPA2 is unaltered
suggesting that aRPA is expressed at higher level in HD brain tissues. Huntington
diseases and myotonic dystrophy and 12 other progressive degenerative are caused by
expansion of the gene-specific trinucleotide CAG/CTG repeat sequence (200-202). The
expanded repeats are unstable, and as individuals with disease age, somatic CAG
expansion will continue and contribute to the disease progression (200,201). The
expansion of CAG repeats can occur during either DNA replication or repair, where the
slipped-DNA is formed after misalignment of the repeats near a replication fork or on
nicked DNA during repair (203,204). These slipped structures can form a number of
conformations because the repeats are able to self-anneal to form a variety of partially
duplex structures. The slipped-DNA structures containing 1-3 repeats are repaired by
mismatch repair (MMR)(201,205,206). Large slipped structures containing more than 3
repeats are repaired by a mechanism that is independent of MMR and nucleotide excision
proteins (207-209).
The ability of aRPA and RPA to support repair of mutagenic slipped-DNA
structures at the CAG/CTG repeats was assessed in vitro in collaboration with Dr.
Christopher Pearson’s lab (Hospital for Sick Children, Toronto, Canada). Even though
RPA is not necessary for repair, the presence of RPA and aRPA was able to enhance the
correct repair and processing of slipped-DNA. However, aRPA was found to inhibit
repair when present at high levels (which approximated the aRPA:RPA ratio in HD
146
brains). Biochemical binding assays were then carried out to examine the binding activity
of RPA and aRPA to slipped-CTG/CAG repeat substrates. These studies showed that two
molecules of RPA but only one molecule of aRPA bound to these slipped DNAs. We
hypothesized that the difference in RPA and aRPA binding is related to the unwinding
activity of the complexes. To test this hypothesis, I assessed the helix destabilization
activity of both forms of RPA. I found that RPA unwound the bubble substrate more
efficiently than aRPA (Figure.AI.12). Taken together, this data suggests that RPA is
better able to destabilize hairpins and slip-outs than aRPA and thus can generate longer
stretches of ssDNA for further RPA binding and repair.
Discussion
Knockdown and reconstituted studies in HeLa cells demonstrated that RPA4 is
unable to support S-phase progression. This result is consistent with previous in vitro
studies that showed that aRPA does not function in SV40 replication. My in vivo studies
suggest that aRPA is able to function in NER, but probably not as efficiently as RPA. It
remains to be determined how well aRPA can function in repairing other types of DNA
damage, such as DSBs, in cells.
To try to control when cells expressed RPA4, I made Tet-off inducible lentiviral
viruses. Transduced cells that showed regulated GFP-RPA2 and GFP-RPA4 were used to
generate stable cells lines, but only a small percentage of the puromycin resistant cells
showed inducible GFP expression.
Because of the lack of success using a lentiviral gene delivery system, I will
consider using plasmid transfection and selection to try to make stable cell lines. I
already have pFIV3.2TREmcs puro GFP-RPA2 and GFP-RPA4 plasmids, which were
shown to express functional GFP-RPA2 and RPA4 in RPA2-depleted cells (Figure 4.6).
By using linearized plasmids, I could minimize the changes disrupting the coding region
of RPA2 or RPA4 upon integration (210). If DNA sequences in pFIV3.2 TRE lentiviral
147
vector affected integration, I also can clone GFP-RPA2 and RPA4 and puromycin
selectable marker into pTRE-tight plasmid obtained from the commercially available Tetoff advanced inducible gene expression system (Clonetech).
The reason that only small percentage of the puromycin resistant cells showed
inducible GFP expression is likely that there is a strong negative selection for RPA4. It
should be noted that I also observed few cells expressing RPA2 with these viruses. This
suggests that whatever was preventing efficient expression; it was affecting both RPA2
and RPA4. Previous studies in our lab with viruses with constitutive promoters also
showed poorer than expected expression of wild type or RPA mutants with modest
phenotypes. Thus, there may be general selection against cells expressing elevated levels
of RPA genes.
This selection could occur by either through a growth selection (slower growth of
RPA2 or RPA4 expressing cells) or by some type of genetic silencing occurring over
time (of either the RPA4 promoter or the TRE promoter). It is intriguing that loss of
expression is occurring even after puromycin selection and long periods of growth under
the repressed (Dox plus) conditions. Under repressed conditions, there should be only
very low levels of expression of exogenous genes carried by these viruses. This suggests
that even low levels of overexpression of RPA genes may be deleterious. If the Tet-off
system is not good for RPA4 induction, other inducible system such as Cre-loxP system
may be used. The goal of these studies was to obtain several homologous populations of
inducible cells. Such regulated RPA4 expression will help us to address the effect of
RPA4 expression level in cellular DNA damage repair and proliferation.
In collaboration with the Pearson lab, we have been exploring the role of aRPA in
repair of the expansion of CAG/CTG repeat sequence. The expansion of number of
CAG/CTG repeats is found in patients with HD, myotonic dystrophy (DM1) and 12 other
progressive degenerative diseases. They have shown that aRPA level was elevated 6-to 8fold in HD brain compared to control brains. In vitro studies suggested that high level of
148
RPA4 reduced repair of slipped-DNA structures and that this could lead to CAG/CTG
expansion.
I carried out studies to test whether altered DNA interactions could be causing
aRPA to inhibit repair of slipped-DNA. It is known that slipped DNAs can adopt
different forms because the repetitive sequences can anneal together to form partially
duplex DNA structures. Both DNA-binding and helix destabilization assays suggested
that aRPA is not as efficient as RPA at melting secondary DNA structures. Such melting
is needed in CAG/CTG tracts to generate uniform ssDNA and facilitate repair. The next
step of these studies will be to apply the ensemble binding assays, the helix
destabilization assay, and single molecule techniques described in the previous chapters
to study interaction of aRPA with different forms of slipped DNA structures. These
studies will allow us determine how affinity of aRPA and/or the melting activity of aRPA
affect its function in repairing slipped DNA.
The in vitro and in vivo studies so far have supported the role of aRPA in DNA
repair in non-dividing cells. Ongoing studies will be targeted at determining the
distribution of RPA in human tissues and the significance of aRPA expression in humans.
The RT-PCR analysis was conducted to compare RPA4 mRNA level in transformed cells
and in normal human tissues. RPA4 expression is very low in transformed cells while
RPA4 expression in normal tissues was over 100 times greater (unpublished result from
Cathy Hass’s thesis). This suggests that RPA4 expression has negative effect for
transformed cells and is suppressed during transformation. It has been found that
treatment of transformed cells with epigenetic modulators, 5’ azacytidine (5’ azaC) and
trichostatin A (TSA), caused a significant increase in RPA4 mRNA level in cultured cells
(unpublished result from Cathy Hass’s thesis). This suggests that RPA4 is epigenetically
silenced in transformed cells, and that the gene can be re-activated by changing these
epigenetic markings. In the future, we would like to determine whether the epigenetic
silencing regulates the expression of RPA in specific human tissues. These studies will
149
allow us to understand how RPA4 level is regulated and significance of RPA4 expression
in different tissues.
150
Figure AI.1. Human RPA2 homologue: RPA4.
The domain arrangements of RPA4 subunit as compared to that of RPA2. DBD=
DNA binding domain,
151
Figure AI.2. RPA4-expressing cells cannot progress through S-phase.
HeLa cells were transfected with RPA2 siRNA and where indicated GFP-tagged
RPA2 and RPA4 vector. At 72 hour after mock or siRNA transfection, cells were
synchronized with 5 µg/mL aphidicolin for 24 h, then released into aphidicolin-free
media. DNA contents were analyzed by flow cytometry at 0, 8 and 24 hours after
release. The number of cells with different DNA contents is plotted. Non-GFP expressing
cells are shown in the mock and knockdown sample (blue line). Only the GFP-expressing
populations were included for the exogenous RPA2 and RPA4 samples (red line).
152
153
Figure AI.3. Co-localization of RPA4 forms with γH2AX.
Cells were treated with RPA2 siRNA and transfected with indicated GFP-RPA
fusion expression plasmid (RPA2, RPA4). Cells were grown in the presence of 10 uM
4NQO for 3 hours, extracted and then prepared for immune-fluorescence as described in
“materials and methods”. Cells were stained with rabbit anti-γH2AX (phosphor-ser139)
and detected using Texas Red-X goat anti-rabbit (IgG) (Invitrogen). Chromatinassociated indicated forms of RPA were visualized using intrinsic GFP and nuclei (DNA)
were visualized with DAPI staining.
154
155
Figure AI.4. RPA4 can partially mediate NER repair.
HeLa cells were grown on coverslips and knockdown and reconstitution of RPA2
and RPA4 was performed as described in Methods. Where indicated, cells were treated
with 0.05 µM 4NQO for 3 hours, washed and allowed to recover for 24 hours. Presence
of DNA damage was monitored by γH2AX. Cells were fixed and visualized by confocal
microscopy either before damage, immediately after 4NQO treatment, or after the 24hour recovery. The intensity of γH2AX staining within individual nuclei was measured
using ImageJ. More than 20 nuclei were counted for each condition. Statistical analysis
on difference between treated, recovery versus untreated in each samples is done using
ANOVA. Cell treatments were as indicated. N=nondamage, D=damage, R=recovery or
rescued. K=knockdown.
156
157
Figure AI.5. Using XPA as NER damage marker is unable detect recovery from 4NQO
damage.
HeLa cells were grown on coverslips and knockdown and reconstitution of RPA2
and RPA4 was performed as described in Methods. Cells were treated with 0.05 µM
4NQO for 3 hours and allowed to recover for 24 hours. The presence of DNA damage
was monitored by quantitating chromatin associated XPA. The intensity of XPA staining
within individual nuclei was measured using ImageJ. More than 20 nuclei were counted
for each sample. Cell treatments are as indicated. N=nondamage, D=damage,
R=recovery or rescued. K=knockdown.
158
159
Figure AI.6. Localization of chromatin-bound XPA after UV and 4NQO damage.
(A) HeLa cells were transfected with RPA2 siRNA and where indicated GFPtagged RPA2 or RPA4 vectors. At 96 hours post-transfections of siRNA, cells were
treated with 10 µM 4NQO for 3 hours. Chromatin fractions were isolated and XPA was
detected using rabbit polyclonal XPA antibody and anti-rabbit HRP. H3 is the loading
control for chromatin-bound proteins. (B) Cells are either UV irradiated for the indicated
intensity or treated with 0.1 or 1 µM 4NQO. XPA localization was examined one hour
after UV irradiation or three hour after 4NQO treatment and at 24 hours after removing
UV and 4NQO. (C) Mock-treated cells, RPA2 knockdown cells and reconstituted cells
were treated with 5 J UV and allowed to recovery for 24 hours. Chromatin-bound XPA
for each sample was determined.
160
A
B
C
161
Figure AI.7. Characterization of the TRE lentiviral vectors with inducible GFP-RPA2
and GFP-RPA4 expression.
(A) Induction of GFP-expression in cells after removing Dox. HeLa tet-off cells
were grown in medium containing Dox, and were transfected with indicated vectors. 24
hours after transfection, cells were either maintained in Dox medium or transferred to
Dox free medium. 72 hours after transfection, the populations of cells with GFP
expression were measured by flow cytometry. (B) Cell-cycle phenotypes for induced
exogenous RPA2 and RPA4 constructs. The DNA content of GFP-positive (RPA2- or
RPA4-experssing) cells was plotted as histogram. The DNA content of GFP-negative
cells for mock and RPA2 siRNA transfected cells was plotted as histogram.
162
A
B
163
Figure AI.8. Characterization of inducible lentivirus in HeLa Tet-off cells.
(A) Cells infected with virus showed inducible GFP expression in the absence of
Dox. Cells were either mock treated or infected with inducible lentivirus carrying GFPRPA2 or GFP-RPA4. Infected cells were grown in minus Dox or plus Dox media for 48
hours. GFP induction is measured by flow cytometry. (B) Induction of a pool of inducible
cells over time. Cells were infected with lentivrus carrying indicated plasmids. After the
infected cells became resistant to puromycine selection, colonies of cells were collected
as a pool. A pool of cells expressing RPA2 and RPA4 were either kept at medium
containing Dox or grew in Dox free medium for the number of days indicated.
Percentage of GFP expressing cells was analyzed by flow cytometry.
164
A
B
165
Figure AI.9.The induction of RPA4 is toxic to replicating cells.
One colony from puro-resistant cells that were infected with inducible GFP-RPA2
(upper) and GFP-RPA4 (lower) lentivirus were grown in six-well plates. The cells from
same colony were grew at plus Dox (1µg/ mL) medium or minus Dox medium for the
indicated days and harvested. Cells expressing GFP were analyzed by Flow cytometry.
The induction of GFP, showing as percent of GFP expressing cells for indicated days was
plotted as shown below.
166
Figure AI.10. RPA4 staining pattern in colon crypt cryosection.
Blue, Nuclear staining with DAPI; green, RPA1 immunofluorescence with antiRPA1 (2H10) 1:2500 and Alexa Fluor 488 (1:3000); Red, RPA4 immunofluorescence
with anti-RPA4 serum (1:5000) and anti-sheep Texas Red (1:3000). ImageJ was used to
process the image.
167
Figure AI.11. Characterization of affinity purified anti-RPA4 antibody using western
blot.
The specificity of anti-RPA4 antibody was exam and compared before (A) and
after (B) the affinity purification.
168
A
B
169
Figure AI.12. RPA and aRPA melt slipped-DNA differently.
DNA helix destabilization assays were carried out. The concentration (nM) of
RPA or aRPA used for each reaction is indicated. 5’ Cy3 labeled 20 nt bubble (6nM) was
used as substrate. The boiled 20 nt bubble substrate is the positive control to show
mobility of the denatured ssDNA.
170
APPENDIX II
EXPRESSION AND ANALYSIS OF BIOTINYLATED RPA3 IN
MAMMALIAN CELLS
Introduction
Cells are most vulnerable to DNA damage during replication because during
replication, damage will result in replication fork stalling. If left unrepaired, DNA lesions
will eventually turn into detrimental double strand breaks (DSBs). Lesions must be either
repaired or bypassed before replication can be resumed. RPA helps coordinate replication
and repair after DNA damage. Studies have found that phosphorylation of RPA is needed
for recovery from damage and fork restart (211).
RPA2 undergoes cell cycle-regulated phosphorylation by the cyclin-dependent
kinase (CDKs) family of kinases (212). Upon DNA damage, RPA2 is phosphorylated by
the phosphoinositide 3 kinase-related kinase families of kinases, including ATM (ataxiatalangiectasia mutated), ATR (ATM and Rad3-related) and DNA-PK (DNA-dependent
protein kinase) (213-215). While ATM and ATR are known to be involved in checkpoint
signaling and DNA-PK is involved in non-homologous end joining (NHEJ) (216).
Multiple sites at N-terminus of RPA2 are phosphorylated. Ser23 and Ser29 are CDK
consensus sites, and are phosphorylated by cyclin A-Cdk2 and cyclin B-Cdk1 during
DNA replication and mitosis (217-219). Ser33 is the primary substrate for ATR and Ser4,
Ser8 and Thr21 are substrates for ATM and DNA-PK (111,215,220). Distinct RPA2
phosphorylation pathways are mediated by PIKKs with overlapping sites of
phosphorylation that vary with types of damage reagents and cell cycle phases (97). Also,
phosphorylation of certain RPA2 residues requires prior phosphorylation of other
residues (97). For example, in cells treated with camptothecin (CPT), a topoisomerase I
inhibitor, RPA2 is initially phosphorylated by ATR at Ser33 and this phosphorylation
171
subsequently stimulates phosphorylation by the cyclin–Cdk and DNA-PK to yield the
hyperphosphorylation (211,221).
Studies have found that phosphorylation of RPA2 changes interaction with partial
duplex DNA and proteins (222,223). The cellular functions of RPA2 phosphorylation in
DNA damage repair are still largely unknown. We have shown that phosphorylation of
RPA2 support normal S phase progression in undamaged HeLa cells and DNA
replication in vitro (224). Under replication stress, RPA2 phosphorylation can stimulate
DNA synthesis and prevent ssDNA accumulation (192,211). RPA phosphorylation also
has been suggested to promote homologous recombination after replication arrest (225).
This Appendix describes initial experiments to examine how phosphorylation
regulates RPA function in DNA repair in vivo using “a single molecule sorting”. This
method was developed in Maria Spies’ lab and can be used to examine the function of in
vivo post-translational modified RPA2 (146). Using this technique allows me to study
activities of the endogenously modified form and unmodified RPA extracted from human
cells simultaneously with only a small amount of protein. In the single molecule sorting,
cells expressing biotinylated RPA are exposed to DNA damaging agents and
phosphorylated RPA is pulled down to the neutravidin coated PEG slides. After
immobilizing the biotinylated RPA, Cy3 labeled oligonucleotide is first added to the
chamber and DNA binding monitored (Figure. AII.1). Then the DNA is washed way and
antibodies to RPA2 or PhosphoRPA2 introduced (Figure.AII.1). These antibodies will
either be directly labeled with Cy3 or Cy5 or labeled secondary antibodies will be used to
localize RPA and phosphorylated RPA. By using this method, we can determine the
kinetics of DNA binding and protein interactions of naturally phosphorylated RPA in
cells. This will lead to a better understanding of how phosphorylation regulates RPA
activity in DNA damage and fork restart.
To make biotinylated RPA, I made constructs to express biotinylated RPA in
mammalian cells. The expression of biotinylated RPA was confirmed by
172
immunoprecipitation. I also tested the specificity of a phospho-site specific antibody to
phosphoRPA2 s33 by both western blot and single molecule analysis. My initial studies
with the recombinant biotinylated RPA showed that phosphoRPA2 antibody was able to
specifically recognize the in vitro phosphorylated RPA.
Materials and Methods
Construction of constructs to express biotinylated RPA in
mammalian cells
We designed DNA sequences that contain the BirA biotin ligase sequence and
FLAG epitope at the N-terminus of RPA3 coding sequence. The DNA sequences, biotin
Flag-tagged RPA3, were ordered from GenScript, where codon was optimized to make it
the synthetic gene efficiently express in human. The biotin Flag-tagged RPA3 fragment
was cut from pUC57 biotin Flag-tagged RPA3 plasmid with XhoI/ KpnI and BamHI and
was cloned into the mammalian vector pEF6/Myc vector as well as pEGFP-C1 vector.
Biotinylated RPA expression in mammalian cells and
purification
Methods for expressing and purifying biotinylated proteins in mammalian cells
were previously described (146). Briefly, cells were transiently transfected with pEGFP
biotin FLAG-RPA3 plasmid. The transfection efficiency was measured by the percentage
of cells expressing GFP. Then, the HEK293T cells were grown in T150 flask and cotransfected with pEF6 biotin FLAG-RPA3 and pcDNA3-BirA (obtain from Spies lab) at
1:1 ratio. Biotin (Sigmal-Aldrich) was added into medium to a final concentration of 100
µM. Cells were harvested 72 hours after transfection. Cells were washed with phosphatebuffered saline, re-suspended in the ice-cold lysis buffer (50 mM HEPES (pH 7.4), 250
mM NaCl, 1mM EDTA, 0.1% Tween 20, 10% glycerol, 1 mM PMSF and cOmplete
protease inhibitors cocktail (Roche)) and incubated at 4°C for 30 minutes. The cells were
173
disrupted by two 15-second pulses of sonication using an Ultrasonics W-22F cell
disruptor at setting 7. Following sonication, the cell lysates are either centrifuged to
purify biotinylated RPA or stored at -80°C until processed further. After centrifugation
(13000 rpm for 10 minutes at 4°C), clarified cell lysate was mixed with anti-FLAG M2
magnetic beads (Sigma-Aldrich) equilibrated with lysis buffer and then incubated at 4°C
for 2 hours with rotation. Beads were then washed with lysis buffer, re-suspended in the
elution buffer (lysis buffer with 150 µg/ml of 3X FLAG peptides (Sigmal-Aldrich) and
incubated at 4°C for 30 minutes. Eluted proteins were immediately divided into small
aliquots and preserved at -80°C.
Sorting phosphorylated RPA and non-phosphorylated RPA
The single molecule sorting was carried out in a flow experiment in the openended fluid chamber (146,226). This chamber allows change of solution during
experiment without any impact on the slide. First, solution containing the biotinylated
RPA, biotinylated phosphorylated RPA or cell extracts were introduced to the chamber,
followed by washes to remove non-biotinylated proteins. Then, solution containing
fluorescently labeled DNA was infused in the chamber, and the binding assays were
carried out. After the binding assay, fluorescently labeled DNA was removed with wash
buffer (50 mM Tris-HCl (pH 7.5), 1M NaCl, 100 ng/ µl BSA). Next, wash buffer was
exchanged with the binding buffer (50 mM Tris-HCl (pH 7.5), 100 mM NaCl, 5 mM
MgCl, 100 ng/µl BSA) containing antibodies (4 nM of mouse anti-RPA2, 4 nM of mouse
anti-RPA1, 4 nM of rabbit anti-phosphoRPA2 s33 (Bethyl)). After 5 minutes of
incubation, the primary antibody solutions were replaced with binding buffer to removed
unbound primary antibodies. Then diluted secondary antibodies (1 nM of Cy3 anti-mouse
and 1 nM of Cy5 anti-rabbit) in binding buffer were infused into the chamber. After 5
minutes of incubation, the chambers were washed with binding buffer to removed
unbound secondary antibodies. Trajectories originated from phosphorylated RPA were
174
distinguished from non-phosphorylated RPA because of the presence of Cy5 signal. The
selectivity of anti-phosphoRPA2 s33 was confirmed by using western blotting and
smTIRF microscopy.
Results
Construction of mammalian plasmid that express
biotinylated RPA3 in mammalian cells
To express biotinylated RPA in mammalian cells, we designed DNA sequence
that has the BirA biotin ligase sequence and FLAG epitope at N-terminus of RPA3
coding sequence. It is expected that endogenous RPA1 and RPA2 subunits will form
complex with biotinylated RPA3 and will be pulled down together on the neutravidin
coated PEG slide. FLAG-tagged biotinylated RPA can be purified from cell extract using
Anti-FLAG M2 magnetic beads if needed.
I made biotin FLAG-RPA3 in both the mammalian vector pEF6 vector as well as
pEGFP vector. I initially confirmed that U2OS cells transfected with pEGFP biotin
FLAG-RPA3 plasmid expressed GFP-RPA3 (30 % of the cells expressed GFP) by flow
cytometry. Next I transfected HEK293T cells with pEF6 biotin FLAG-RPA3 and
pcDNA-BirA plasmid to obtain biotinylated RPA complex. HEK293T cells have been
used previously for making biotinylated proteins in single-molecule sorting(146). In
small-scale expression experiments (in a six-well plate), no biotinylated RPA was
detected by western blotting with anti-biotin antibody. I then scaled up the transformation
(in a T150 flask) and did a pull-down with the streptavidin agarose beads. The
streptavidin agarose beads should pull down biotinylated RPA3, in complex with the
RPA1 and RPA2 subunits. In this case, both RPA2 and FLAG-tagged RPA3 could be
detected (Figure.AII.2). The positive control was cells transfected with pcDNA3 FLAGFBH1-BAP plasmid, which is known to express Flag-tagged biotinylated FBH1 protein
(146). When a pull down was done from cells transfected with pcDNA3 FLAG-FBH1-
175
BAP plasmid using streptavidin agarose beads, no RPA2 was immunoprecipitated in the
control (Figure.AII.2). I re-probed the same western blot with anti-FLAG antibody, and I
could detect FLAG-tagged RPA3 but not FLAG-tagged FBH1 (Figure.AII.2). FBH1 is
high molecular weight protein, which is around 150 kDa and I believe that transfer or
blotting was an issue. Hence, I showed that we could make biotinylated RPA in
mammalian cells and these biotinylated-RPA are also Flag-tagged.
Detection of phosphorylated biotinylated RPA in cells
In order to determine whether phosphorylated RPA can be pull down with
biotinylated RPA3, I also examined extracts from pEF6 biotin FLAG-RPA3 transfected
cells that had been treated with Camptothecin (Cpt). After pull-down with streptavidin
beads, I expected to observe phosphorylated biotinylated RPA in these cells. Both the
input lysates and supernatants showed phosphorylated RPA2 in CPT-treated cells when
anti-RPA2 antibody was used; phosphorylated RPA2 has a different mobility and
appeared as higher molecular bands above RPA2 (Figure.AII.2). However, no
phosphorylated biotinylated RPA was detected (Figure.AII.2). This might be due to the
low abundance of phosphorylated RPA or a technical issue. “Single-molecule sorting”
detects individual phosphorylated molecules so even if there are only low levels of
phosphorylated biotinylated RPA after DNA damage, this method can be used to detect
phosphorylated RPA.
PhosphoRPA2 (S33) antibody is specific to phosphorylated
RPA
While these studies were being carried out, I wanted to confirm that the
phosphoRPA2 antibody is specific and also can be used in single molecule experiment.
So I damaged cells with camptothecin (CPT), and made the whole cell extracts of
damaged cells and non-damaged cells. These were analyzed by western blotting using
phosphoRPA2 (S33) antibody. Recombinant RPA and in vitro phosphorylated RPA using
176
SV40 replication system were used as controls (Figure.AII.3B). The anti-phosphoRPA2
does not recognize non-phosphorylated RPA2. There was an increased in RPA2
phosphorylation upon DNA damage detected by RPA2 antibody and phosphoRPA2
(S33) antibody (Figure.AII.3B).
Phosphorylated RPA2 can be detected in single molecule
experiment
To determine whether phosphoRPA2 (S33) can be used in the single molecule
experiment, I phosphorylated biotinylated RPA in vitro using the SV40 replication
system. The in vitro phosphorylated RPA is immobilized to the neutravidin coated PEGslide. Biotinylated RPA was used as non-phosphorylated RPA control. Chambers with
biotinylated RPA and phosphorylated PRA are incubated with primary antibody to RPA
and phosphoRPA and Cy3 and Cy5 labeled secondary antibody (Figure.AII.3A). Cy3
signals should come from RPA2 and Cy5 signal should come from phophoRPA2. In
chamber with non-phosphorylated RPA, there were many signals in Cy3 channel and
very little signal in Cy5 channel (Figure.AII.3C). In chamber with phosphorylated RPA, I
saw signals from both Cy3 channel and Cy5 signal (Figure.AII.3C). Both RPA2 antibody
and phosphoRPA2 antibody should recognize phosphorylated RPA2, so I expected to see
a co-localization of Cy3 and Cy5 signal for phosphorylated RPA. I examined each
trajectory of a single RPA molecule and counted 200 trajectories from each RPA
chamber and phosphorylated RPA chamber. I categorized those trajectories in Cy3 only,
Cy5 only and both Cy3 Cy5 FRET. The result is shown in Figure.A.3D. Most signals in
RPA chamber are from Cy3 and there are no molecules with Cy5 only or FRET. In the
phosphorylated RPA chamber, I had Cy5 only molecules, Cy3 only molecules and also
molecules with FRET (both labels). Only some RPA is phosphorylated using this in vitro
system, which explain Cy3 only molecules. For those Cy5 only molecules, they are likely
phosphorylated RPA but are not recognized by RPA2 antibody because of competition
177
between antibodies. Alternatively, the antibodies could be limiting or there could be
some non-specific binding of phosphoRPA antibody. I will further determine the
specificity RPA2 (S33) antibody by doing lambda phosphatase treated controls and using
cells extracts in addition to purified biotinylated RPA.
Discussion
The phosphorylation of RPA has a potential regulatory role for RPA function in
different DNA metabolic pathways. Phosphorylation of RPA is known to regulate its
biochemical activities and interactions with other proteins. A mimetic of phosphorylated
RPA has been made for biological and chemical studies; however, there are always
concerns that phosphate-mimetics may not function the same as phosphorylation. The
alternative is to separate the endogenously phosphorylated RPA from unmodified
counterparts. Due to the availability of protein and the fact that only a small fraction of
RPA is modified in cells, it has been technically challenging to study endogenously
phosphorylated RPA. Here, we showed that “single molecule sorting” could be used to
detect endogenous RPA phosphorylation in mammalian cells together with the unmodified RPA. We were able to express biotinylated RPA in mammalian cells and to
optimize the condition for detecting in vitro phosphorylated RPA in the single molecule
experiment.
The next step will be to visualize the endogenously modified RPA from cell
extract expressing the biotinylated RPA. It is expected that majority of RPA will be unmodified in cells. We would like to estimate the percentage of phosphorylated RPA upon
DNA damage. Also, by using this method, we will be evaluating the activities of the
phosphorylated and non-phosphorylated RPA extracted from human cells in the same
experiment. The kinetics of binding to different fluorescently labeled DNA substrates
mimicking the partial duplex DNA structures present at the site of DNA damage could be
examine. Recombinant forms of RPA including phosphorylation mimetics can also be
178
examined. These studies will tell us how phosphorylation alters interactions of RPA with
different forms of DNA. They will also provide more insights into how phosphorylation
regulates RPA function in DNA damage repair.
179
Figure AII.1. Schematic representation of “single-molecule cell sorting”.
(A) A mixed population of phosphorylated RPA and non-phosphorylated RPA is
tethered to the slide surface. The binding of Cy3-labeled DNA to RPA is first examined.
(B) Antibodies are later added to distinguish phosphorylated RPA from nonphosphorylated RPA. The primary antibodies recognizing either RPA2 or phosphoRPA2
are labeled with Cy3 or Cy3. Non-phosphorylated RPA only exhibits Cy3 signal.
Phosphorylated RPA exhibits both Cy3 and Cy5 signal.
180
A
B
181
Figure AII.2. Detecting biotinylated RPA in mammalian cells.
RPA and biotinylated RPA are loading controls. Cell lysates from cells
transfected with pEF6 biotin FLAG-RPA3 plasmid and pcDND3 FLAG-FBH1-BAP
plasmid were used to pull down biotinylated RPA and FBH1 (using 100 µl of streptavidin
beads). Cells expressing biotinylated RPA were also damaged with 20 µM camptothecin
(CPT). The pull-down biotinylated RPA and FLAG-tagged RPA were detected by RPA2
antibody and Anti-FLAG antibody. 1/20 volume of cell lysates used for pull-down and
1/20 volume of supernatant after pull-down were loaded.
182
183
Figure AII.3. Characterizing phosphoRPA2 (S33) antibody in single molecule
experiment.
(A) The experimental layout of the assay. Chamber 1 has biotinylated RPA.
Chamber 2 has in vitro phosphorylated biotinylated RPA. Both anti-RPA2 and antiphosphoRPA2 (S33) were used. Cy3 labeled secondary antibody recognized RPA2, and
Cy5 labeled secondary antibody recognize phosphoRPA2. (B) PhosphoRPA (S33)
specificity is tested using Western blot. RPA and in vitro phosphorylated RPA are
controls for phosphorylated and non-phosphorylated RPA. Cell extract of cells treated or
not treated with camptothecin (20 µM) were also analyzed by western blot.
PhosphoRPA2 has different mobility from RPA2 on the gel. (C) Single molecule images
of Cy3 and Cy5 channel for chamber 1 and chamber 2 are shown. Cy3 or Cy5 signals
were reflected as black spots on the grey background. (D) 200 trajectories from each
biotinylated RPA chamber and phosphorylated RPA chamber are counted and are divided
in to three categories, Cy5 only, Cy3 only or Cy3 Cy5 FRET. The number of events for
each category is shown.
184
A
B
C
D
185
REFERENCES
1.
Lopez, P., Philippe, H., Myllykallio, H., and Forterre, P. (1999) Identification of
putative chromosomal origins of replication in Archaea. Mol.Microbiol. 32, 883886
2.
Murzin, A. G. (1993) OB(oligonucleotide/oligosaccharide binding)-fold: common
structural and functional solution for non-homologous sequences. Embo J 12,
861-867
3.
Fanning, E., Klimovich, V., and Nager, A. R. (2006) A dynamic model for
replication protein A (RPA) function in DNA processing pathways. Nucleic Acids
Res 34, 4126-4137
4.
Shereda, R. D., Kozlov, A. G., Lohman, T. M., Cox, M. M., and Keck, J. L.
(2008) SSB as an organizer/mobilizer of genome maintenance complexes. Crit
Rev Biochem Mol Biol 43, 289-318
5.
Ha, T., Kozlov, A. G., and Lohman, T. M. (2012) Single-molecule views of
protein movement on single-stranded DNA. Annual review of biophysics 41, 295319
6.
Wold, M. S. (1997) Replication Protein A: A heterotrimeric, single-stranded
DNA-binding protein required for eukaryotic DNA metabolism. Annu Rev
Biochem 66, 61-92
7.
Richard, D. J., Bolderson, E., and Khanna, K. K. (2009) Multiple human singlestranded DNA binding proteins function in genome maintenance: structural,
biochemical and functional analysis. Crit Rev Biochem Mol Biol 44, 98-116
8.
Iftode, C., Daniely, Y., and Borowiec, J. A. (1999) Replication Protein A (RPA):
The eukaryotic SSB. CRC Critical Reviews in Biochemistry 34, 141-180
9.
Ashton, N. W., Bolderson, E., Cubeddu, L., O'Byrne, K. J., and Richard, D. J.
(2013) Human single-stranded DNA binding proteins are essential for
maintaining genomic stability. BMC molecular biology 14, 9
10.
Lei, M., Podell, E. R., and Cech, T. R. (2004) Structure of human POT1 bound to
telomeric single-stranded DNA provides a model for chromosome end-protection.
Nat Struct Mol Biol 11, 1223-1229
11.
Oliveira, M. T., and Kaguni, L. S. (2010) Functional roles of the N- and Cterminal regions of the human mitochondrial single-stranded DNA-binding
protein. PLoS One 5, e15379
12.
Richard, D. J., Bolderson, E., Cubeddu, L., Wadsworth, R. I., Savage, K., Sharma,
G. G., Nicolette, M. L., Tsvetanov, S., McIlwraith, M. J., Pandita, R. K., Takeda,
S., Hay, R. T., Gautier, J., West, S. C., Paull, T. T., Pandita, T. K., White, M. F.,
186
and Khanna, K. K. (2008) Single-stranded DNA-binding protein hSSB1 is critical
for genomic stability. Nature 453, 677-681
13.
Marceau, A. H. (2012) Functions of single-strand DNA-binding proteins in DNA
replication, recombination, and repair. Methods Mol Biol 922, 1-21
14.
Raghunathan, S., Kozlov, A. G., Lohman, T. M., and Waksman, G. (2000)
Structure of the DNA binding domain of E-coli SSB bound to ssDNA. Nature
Struct.Biol. 7, 648-652
15.
Fairman, M. P., and Stillman, B. (1988) Cellular factors required for multiple
stages of SV40 DNA replication in vitro. EMBO J. 7, 1211-1218
16.
Wold, M. S., and Kelly, T. (1988) Purification and characterization of replication
protein A, a cellular protein required for in vitro replication of simian virus 40
DNA. Proc Natl Acad Sci USA 85, 2523-2527
17.
Heyer, W.-D., Rao, M. R. S., Erdile, L. F., Kelly, T. J., and Kolodner, R. D.
(1990) An essential Saccharomyces cerevisiae single-stranded DNA binding
protein is homologous to the large subunit of human RP-A. EMBO J. 9, 23212329
18.
Parker, A. E., Clyne, R. K., Carr, A. M., and Kelly, T. J. (1997) The
Schizosaccharomyces pombe rad11+ gene encodes the large subunit of replication
protein A. Mol.Cell.Biol. 17, 2381-2390
19.
Mitsis, P. G., Kowalczykowski, S. C., and Lehman, I. R. (1993) A single-stranded
DNA binding protein from Drosophila melanogaster: Characterization of the
heterotrimeric protein and its interaction with single-stranded DNA. Biochemistry
32, 5257-5266
20.
Van der Knaap, E., Jagoueix, S., and Kende, H. (1997) Expression of an ortholog
of replication protein A1 (RPA1) is induced by gibberellin in deepwater rice.
Proc.Natl.Acad.Sci.USA 94, 9979-9983
21.
Wadsworth, R. I., and White, M. F. (2001) Identification and properties of the
crenarchaeal single-stranded DNA binding protein from Sulfolobus solfataricus.
Nucleic Acids Research 29, 914-920
22.
Kelly, T. J., Simancek, P., and Brush, G. S. (1998) Identification and
characterization of a single-stranded DNA-binding protein from the archaeon
Methanococcus jannaschii. Proc.Natl.Acad.Sci.USA 95, 14634-14639
23.
Tiranti, V., Rocchi, M., DiDonato, S., and Zeviani, M. (1993) Cloning of human
and rat cDNAs encoding the mitochondrial single-stranded DNA-binding protein
(SSB). Gene 126, 219-225
187
24.
Broderick, S., Rehmet, K., Concannon, C., and Nasheuer, H. P. (2010) Eukaryotic
single-stranded DNA binding proteins: central factors in genome stability. Subcell
Biochem 50, 143-163
25.
Li, Y., Bolderson, E., Kumar, R., Muniandy, P. A., Xue, Y., Richard, D. J.,
Seidman, M., Pandita, T. K., Khanna, K. K., and Wang, W. (2009) HSSB1 and
hSSB2 form similar multiprotein complexes that participate in DNA damage
response. J Biol Chem 284, 23525-23531
26.
Bochkarev, A., and Bochkareva, E. (2004) From RPA to BRCA2: lessons from
single-stranded DNA binding by the OB-fold. Curr Opin Struct Biol 14, 36-42
27.
Bochkareva, E., Korolev, S., Lees-Miller, S. P., and Bochkarev, A. (2002)
Structure of the RPA trimerization core and its role in the multistep DNA-binding
mechanism of RPA. EMBO J 21, 1855-1863
28.
Brosey, C. A., Chagot, M. E., Ehrhardt, M., Pretto, D. I., Weiner, B. E., and
Chazin, W. J. (2009) NMR analysis of the architecture and functional remodeling
of a modular multidomain protein, RPA. J Am Chem Soc 131, 6346-6347
29.
Xu, X., Vaithiyalingam, S., Glick, G. G., Mordes, D. A., Chazin, W. J., and
Cortez, D. (2008) The basic cleft of RPA70N binds multiple checkpoint proteins
including RAD9 to regulate ATR signaling. Mol Cell Biol 28, 7345-7353
30.
Binz, S. K., and Wold, M. S. (2008) Regulatory functions of the N-terminal
domain of the 70-kDa subunit of replication protein A (RPA). J Biol Chem 283,
21559-21570
31.
Haring, S. J., Mason, A. C., Binz, S. K., and Wold, M. S. (2008) Cellular
functions of human RPA1. Multiple roles of domains in replication, repair, and
checkpoints. J Biol Chem 283, 19095-19111
32.
Wyka, I. M., Dhar, K., Binz, S. K., and Wold, M. S. (2003) Replication protein A
interactions with DNA: differential binding of the core domains and analysis of
the DNA interaction surface. Biochemistry 42, 12909-12918
33.
Arunkumar, A. I., Stauffer, M. E., Bochkareva, E., Bochkarev, A., and Chazin,
W. J. (2003) Independent and Coordinated Functions of Replication Protein A
Tandem High Affinity Single-stranded DNA Binding Domains. J Biol Chem 278,
41077-41082
34.
Binz, S. K., Sheehan, A. M., and Wold, M. S. (2004) Replication protein A
phosphorylation and the cellular response to DNA damage. DNA Repair (Amst) 3,
1015-1024
35.
Oakley, G. G., and Patrick, S. M. (2010) Replication protein A: directing traffic at
the intersection of replication and repair. Front Biosci 15, 883-900
188
36.
Gao, H., Cervantes, R. B., Mandell, E. K., Otero, J. H., and Lundblad, V. (2007)
RPA-like proteins mediate yeast telomere function. Nat Struct Mol Biol 14, 208214
37.
Bochkarev, A., Bochkareva, E., Frappier, L., and Edwards, A. M. (1999) The
crystal structure of the complex of replication protein A subunits RPA32 and
RPA14 reveals a mechanism for single-stranded DNA binding. EMBO J 18,
4498-4504
38.
Sakaguchi, K., Ishibashi, T., Uchiyama, Y., and Iwabata, K. (2009) The multireplication protein A (RPA) system--a new perspective. Febs J 276, 943-963
39.
Ishibashi, T., Kimura, S., Furukawa, T., Hatanaka, M., Hashimoto, J., and
Sakaguchia, K. (2001) Two types of replication protein A 70 kDa subunit in rice,
Oryza sativa: molecular cloning, characterization, and cellular & tissue
distribution. Gene 272, 335-343
40.
Ishibashi, T., Kimura, S., and Sakaguchi, K. (2006) A Higher Plant Has Three
Different Types of RPA Heterotrimeric Complex. J Biochem (Tokyo) 139, 99-104
41.
Keshav, K. F., Chen, C., and Dutta, A. (1995) Rpa4, a homolog of the 34kilodalton subunit of the replication protein A complex. Mol.Cell.Biol. 15, 31193128
42.
Haring, S. J., Humphreys, T. D., and Wold, M. S. (2010) A naturally occurring
human RPA subunit homolog does not support DNA replication or cell-cycle
progression. Nucleic Acids Res 38, 846-858
43.
Mason, A. C., Haring, S. J., Pryor, J. M., Staloch, C. A., Gan, T. F., and Wold, M.
S. (2009) An alternative form of replication protein A prevents viral replication in
vitro. J Biol Chem 284, 5324-5331
44.
Kemp, M. G., Mason, A. C., Carreira, A., Reardon, J. T., Haring, S. J., Borgstahl,
G. E., Kowalczykowski, S. C., Sancar, A., and Wold, M. S. (2009) An alternative
form of RPA (aRPA) expressed in normal human tissues supports DNA repair. J
Biol Chem
45.
Mason, A. C., Roy, R., Simmons, D. T., and Wold, M. S. (2010) Functions of
alternative replication protein A in initiation and elongation. Biochemistry 49,
5919-5928
46.
Fan, J., and Pavletich, N. P. (2012) Structure and conformational change of a
replication protein A heterotrimer bound to ssDNA. Genes Dev 26, 2337-2347
47.
de Laat, W. L., Appeldoorn, E., Sugasawa, K., Weterings, E., Jaspers, N. G., and
Hoeijmakers, J. H. (1998) DNA-binding polarity of human replication protein A
positions nucleases in nucleotide excision repair. Genes Dev 12, 2598-2609
189
48.
Iftode, C., and Borowiec, J. A. (2000) 5' --> 3' molecular polarity of human
replication protein A (hRPA) binding to pseudo-origin DNA substrates.
Biochemistry 39, 11970-11981
49.
Bochkarev, A., Pfuetzner, R. A., Edwards, A. M., and Frappier, L. (1997)
Structure of the single-stranded-DNA-binding domain of replication protein A
bound to DNA. Nature 385, 176-181
50.
Blackwell, L. J., and Borowiec, J. A. (1994) Human replication protien A binds
single-stranded DNA in two distinct complexes. Mol Cell Biol 14, 3993-4001
51.
Kim, C., Paulus, B. F., and Wold, M. S. (1994) Interactions of Human Replication
Protein A with Oligonucleotides. Biochemistry 33, 14197-14206
52.
Brosey, C. A., Yan, C., Tsutakawa, S. E., Heller, W. T., Rambo, R. P., Tainer, J.
A., Ivanov, I., and Chazin, W. J. (2013) A new structural framework for
integrating replication protein A into DNA processing machinery. Nucleic Acids
Res 41, 2313-2327
53.
Kumaran, S., Kozlov, A. G., and Lohman, T. M. (2006) Saccharomyces
cerevisiae Replication Protein A Binds to Single-Stranded DNA in Multiple SaltDependent Modes. Biochemistry 45, 11958-11973
54.
Nguyen, B., Sokoloski, J., Galletto, R., Elson, E. L., Wold, M. S., and Lohman, T.
M. (2014) Diffusion of human Replication Protein A along single stranded DNA.
J Mol Biol 21, 00361-00361
55.
Bae, S. H., Bae, K. H., Kim, J. A., and Seo, Y. S. (2001) RPA governs
endonuclease switching during processing of Okazaki fragments in eukaryotes.
Nature.412(6845):456-461,.2001.Jul.26., 456-461
56.
Yuzhakov, A., Kelman, Z., Hurwitz, J., and O'Donnell, M. (1999) Multiple
competition reactions for RPA order the assembly of the DNA polymerase delta
holoenzyme. Embo J 18, 6189-6199
57.
Stewart, J. A., Miller, A. S., Campbell, J. L., and Bambara, R. A. (2008) Dynamic
removal of replication protein A by Dna2 facilitates primer cleavage during
Okazaki fragment processing in Saccharomyces cerevisiae. J Biol Chem
58.
Sancar, A., Lindsey-Boltz, L. A., Unsal-Kacmaz, K., and Linn, S. (2004)
Molecular mechanisms of mammalian DNA repair and the DNA damage
checkpoints. Annu Rev Biochem 73, 39-85
59.
Tapias, A., Auriol, J., Forget, D., Enzlin, J. H., Scharer, O. D., Coin, F.,
Coulombe, B., and Egly, J. M. (2004) Ordered conformational changes in
damaged DNA induced by nucleotide excision repair factors. J Biol Chem 279,
19074-19083
190
60.
Missura, M., Buterin, T., Hindges, R., Hubscher, U., Kasparkova, J., Brabec, V.,
and Naegeli, H. (2001) Double-check probing of DNA bending and unwinding by
XPA-RPA: an architectural function in DNA repair. Embo Journal 20 (13), 35543564
61.
Moser, J., Kool, H., Giakzidis, I., Caldecott, K., Mullenders, L. H., and Fousteri,
M. I. (2007) Sealing of chromosomal DNA nicks during nucleotide excision
repair requires XRCC1 and DNA ligase III alpha in a cell-cycle-specific manner.
Mol Cell 27, 311-323
62.
Mimitou, E. P., and Symington, L. S. (2011) DNA end resection--unraveling the
tail. DNA Repair (Amst) 10, 344-348
63.
Chen, H., Lisby, M., and Symington, L. S. (2013) RPA coordinates DNA end
resection and prevents formation of DNA hairpins. Mol Cell 50, 589-600
64.
Krasner, D. S., Daley, J. M., Sung, P., and Niu, H. (2015) Interplay between Ku
and RPA in the restriction of Exo1-mediated DNA break end resection. J Biol
Chem
65.
San Filippo, J., Sung, P., and Klein, H. (2008) Mechanism of eukaryotic
homologous recombination. Annu Rev Biochem 77, 229-257
66.
Sugiyama, T., and Kantake, N. (2009) Dynamic regulatory interactions of rad51,
rad52, and replication protein-a in recombination intermediates. J Mol Biol 390,
45-55
67.
Eggler, A. L., Inman, R. B., and Cox, M. M. (2002) The Rad51-dependent pairing
of long DNA substrates is stabilized by replication protein A. J Biol Chem 277,
39280-39288
68.
Sneeden, J. L., Grossi, S. M., Tappin, I., Hurwitz, J., and Heyer, W. D. (2013)
Reconstitution of recombination-associated DNA synthesis with human proteins.
Nucleic Acids Res 41, 4913-4925
69.
Xue, X., Raynard, S., Busygina, V., Singh, A. K., and Sung, P. (2013) Role of
replication protein A in double holliday junction dissolution mediated by the
BLM-Topo IIIalpha-RMI1-RMI2 protein complex. J Biol Chem 288, 1422114227
70.
Krejci, L., Altmannova, V., Spirek, M., and Zhao, X. (2012) Homologous
recombination and its regulation. Nucleic Acids Res 40, 5795-5818
71.
Villarreal, D. D., Lee, K., Deem, A., Shim, E. Y., Malkova, A., and Lee, S. E.
(2012) Microhomology directs diverse DNA break repair pathways and
chromosomal translocations. PLoS Genet 8, e1003026
191
72.
Deng, S. K., Gibb, B., de Almeida, M. J., Greene, E. C., and Symington, L. S.
(2014) RPA antagonizes microhomology-mediated repair of DNA double-strand
breaks. Nat Struct Mol Biol 21, 405-412
73.
Cimprich, K. A., and Cortez, D. (2008) ATR: an essential regulator of genome
integrity. Nature reviews 9, 616-627
74.
Bao, S. D., Tibbetts, R. S., Brumbaugh, K. M., Fang, Y. N., Richardson, D. A.,
Ali, A., Chen, S. M., Abraham, R. T., and Wang, X. F. (2001) ATR/ATMmediated phosphorylation of human Rad17 is required for genotoxic stress
responses. Nature.411(6840):969-974,.2001.Jun.21., 969-974
75.
Zou, L., and Elledge, S. J. (2003) Sensing DNA damage through ATRIP
recognition of RPA-ssDNA complexes. Science 300, 1542-1548
76.
Lee, J. H., and Paull, T. T. (2005) ATM activation by DNA double-strand breaks
through the Mre11-Rad50-Nbs1 complex. Science 308, 551-554
77.
Falck, J., Coates, J., and Jackson, S. P. (2005) Conserved modes of recruitment of
ATM, ATR and DNA-PKcs to sites of DNA damage. Nature 434, 605-611
78.
Bartek, J., and Lukas, J. (2003) DNA repair: Damage alert. Nature 421, 486-488
79.
Parrilla-Castellar, E. R., Arlander, S. J., and Karnitz, L. (2004) Dial 9-1-1 for
DNA damage: the Rad9-Hus1-Rad1 (9-1-1) clamp complex. DNA Repair (Amst)
3, 1009-1014
80.
Ellison, V., and Stillman, B. (2003) Biochemical Characterization of DNA
Damage Checkpoint Complexes: Clamp Loader and Clamp Complexes with
Specificity for 5' Recessed DNA. PLoS Biol 1, 231-243
81.
Majka, J., Binz, S. K., Wold, M. S., and Burgers, P. M. (2006) Replication protein
A directs loading of the DNA damage checkpoint clamp to 5'-DNA junctions. J
Biol Chem 281, 27855-27861
82.
Kumagai, A., Lee, J., Yoo, H. Y., and Dunphy, W. G. (2006) TopBP1 activates
the ATR-ATRIP complex. Cell 124, 943-955
83.
Mordes, D. A., Glick, G. G., Zhao, R., and Cortez, D. (2008) TopBP1 activates
ATR through ATRIP and a PIKK regulatory domain. Genes Dev 22, 1478-1489
84.
Dutta, A., Ruppert, J. M., Aster, J. C., and Winchester, E. (1993) Inhibition of
DNA replication factor RPA by p53. Nature 365, 79-82
85.
Choudhary, S. K., and Li, R. (2002) BRCA1 modulates ionizing radiationinduced nuclear focus formation by the replication protein A p34 subunit. J Cell
Biochem 84, 666-674
192
86.
Yang, H., Jeffrey, P. D., Miller, J., Kinnucan, E., Sun, Y., Thoma, N. H., Zheng,
N., Chen, P. L., Lee, W. H., and Pavletich, N. P. (2002) BRCA2 function in DNA
binding and recombination from a BRCA2-DSS1-ssDNA structure. Science 297,
1837-1848
87.
Olson, E., Nievera, C. J., Liu, E., Lee, A. Y., Chen, L., and Wu, X. (2007) The
Mre11 complex mediates the S-phase checkpoint through an interaction with
replication protein A. Mol Cell Biol 27, 6053-6067
88.
Brill, S. J., and Stillman, B. (1991) Replication factor-A from Saccharomyces
cerevisiae is encoded by three essential genes coordinately expressed at S phase.
Genes Dev. 5, 1589-1600
89.
Smith, J., and Rothstein, R. (1995) A mutation in the gene encoding the
Saccharomyces cerevisiae single-stranded DNA-binding protein Rfa1 stimulates a
RAD52-independent pathway for direct-repeat recombination. Mol.Cell.Biol. 15,
1632-1641
90.
Philipova, D., Mullen, J. R., Maniar, H. S., Gu, C., and Brill, S. J. (1996) A
hierachachy of SSB protomers in Replication Protein-A. Genes Dev. 10, 22222233
91.
Santocanale, C., Neecke, H., Longhese, M. P., Lucchini, G., and Plevani, P.
(1995) Mutations in the gene encoding the 34 kDa subunit of yeast replication
protein A cause defective S phase progression. J Mol Biol 254, 595-607
92.
Wang, Y., Putnam, C. D., Kane, M. F., Zhang, W., Edelmann, L., Russell, R.,
Carrion, D. V., Chin, L., Kucherlapati, R., Kolodner, R. D., and Edelmann, W.
(2005) Mutation in Rpa1 results in defective DNA double-strand break repair,
chromosomal instability and cancer in mice. Nat Genet 37, 750-755
93.
Hass, C. S., Gakhar, L., and Wold, M. S. (2010) Functional characterization of a
cancer causing mutation in human Replication Protein A. Molecular Cancer
Research 8, 1017-1026
94.
O'Driscoll, M., Dobyns, W. B., van Hagen, J. M., and Jeggo, P. A. (2007)
Cellular and clinical impact of haploinsufficiency for genes involved in ATR
signaling. Am J Hum Genet 81, 77-86
95.
Outwin, E., Carpenter, G., Bi, W., Withers, M. A., Lupski, J. R., and O'Driscoll,
M. (2011) Increased RPA1 gene dosage affects genomic stability potentially
contributing to 17p13.3 duplication syndrome. PLoS Genet 7, e1002247
96.
Toledo, L. I., Altmeyer, M., Rask, M. B., Lukas, C., Larsen, D. H., Povlsen, L.
K., Bekker-Jensen, S., Mailand, N., Bartek, J., and Lukas, J. (2013) ATR
prohibits replication catastrophe by preventing global exhaustion of RPA. Cell
155, 1088-1103
193
97.
Liu, S., Opiyo, S. O., Manthey, K., Glanzer, J. G., Ashley, A. K., Amerin, C.,
Troksa, K., Shrivastav, M., Nickoloff, J. A., and Oakley, G. G. (2012) Distinct
roles for DNA-PK, ATM and ATR in RPA phosphorylation and checkpoint
activation in response to replication stress. Nucleic Acids Res 40, 10780-10794
98.
Stauffer, M. E., and Chazin, W. J. (2004) Physical interaction between replication
protein A and Rad51 promotes exchange on single-stranded DNA. J Biol Chem
279, 25638-25645
99.
Mer, G., Bochkarev, A., Gupta, R., Bochkareva, E., Frappier, L., Ingles, C. J.,
Edwards, A. M., and Chazin, W. J. (2000) Structural basis for the recognition of
DNA repair proteins UNG2, XPA, and RAD52 by replication factor RPA. Cell
103, 449-456
100.
Arunkumar, A. I., Klimovich, V., Jiang, X., Ott, R. D., Mizoue, L., Fanning, E.,
and Chazin, W. J. (2005) Insights into hRPA32 C-terminal domain--mediated
assembly of the simian virus 40 replisome. Nat Struct Mol Biol 12, 332-339
101.
Jiang, X., Klimovich, V., Arunkumar, A. I., Hysinger, E. B., Wang, Y., Ott, R. D.,
Guler, G. D., Weiner, B., Chazin, W. J., and Fanning, E. (2006) Structural
mechanism of RPA loading on DNA during activation of a simple pre-replication
complex. Embo J 25, 5516-5526
102.
Vaithiyalingam, S., Warren, E. M., Eichman, B. F., and Chazin, W. J. (2010)
Insights into eukaryotic DNA priming from the structure and functional
interactions of the 4Fe-4S cluster domain of human DNA primase. Proc Natl
Acad Sci U S A 107, 13684-13689
103.
Guler, G. D., Liu, H., Vaithiyalingam, S., Arnett, D. R., Kremmer, E., Chazin, W.
J., and Fanning, E. (2011) Human DNA Helicase B (HDHB) binds to replication
protein A and facilitates cellular recovery from replication stress. J Biol Chem
104.
Jackson, D., Dhar, K., Wahl, J. K., Wold, M. S., and Borgstahl, G. E. (2002)
Analysis of the human replication protein A:Rad52 complex: evidence for
crosstalk between RPA32, RPA70, Rad52 and DNA. J Mol Biol 321, 133-148
105.
Bochkareva, E., Kaustov, L., Ayed, A., Yi, G. S., Lu, Y., Pineda-Lucena, A.,
Liao, J. C., Okorokov, A. L., Milner, J., Arrowsmith, C. H., and Bochkarev, A.
(2005) Single-stranded DNA mimicry in the p53 transactivation domain
interaction with replication protein A. Proc Natl Acad Sci U S A 102, 1541215417
106.
Feldkamp, M. D., Mason, A. C., Eichman, B. F., and Chazin, W. J. (2014)
Structural analysis of RPA recruitment of the DNA damage response protein
SMARCAL1. Biochemistry 53, 3052-3061
107.
Borgstahl, G. E. O., Brader, K., Mosel, A., Liu, S., Kremmer, E., Goettsch, K. A.,
Kolar, C., Nasheuer, H.-P., and Oakley, G. G. (2014) Interplay of DNA damage
194
and cell cycle signaling at the level of human replication protein A. DNA Repair
21, 12-23
108.
Din, S., Brill, S. J., Fairman, M. P., and Stillman, B. (1990) Cell-cycle-regulated
phosphorylation of DNA replication factor A from human and yeast cells. Genes
& Development 4, 968-977
109.
Marechal, A., and Zou, L. (2014) RPA-coated single-stranded DNA as a platform
for post-translational modifications in the DNA damage response. Cell research
110.
Henricksen, L. A., Carter, T., Dutta, A., and Wold, M. S. (1996) Phosphorylation
of human Replication Protein A by the DNA-dependent Protein Kinase is
involved in the modulation of DNA replication. Nucleic Acids Res 24, 3107-3112
111.
Olson, E., Nievera, C. J., Klimovich, V., Fanning, E., and Wu, X. (2006) RPA2 is
a direct downstream target for ATR to regulate the S-phase checkpoint. J Biol
Chem 281, 39517-39533
112.
Ashley, A. K., Shrivastav, M., Nie, J., Amerin, C., Troksa, K., Glanzer, J. G., Liu,
S., Opiyo, S. O., Dimitrova, D. D., Le, P., Sishc, B., Bailey, S. M., Oakley, G. G.,
and Nickoloff, J. A. (2014) DNA-PK phosphorylation of RPA32 Ser4/Ser8
regulates replication stress checkpoint activation, fork restart, homologous
recombination and mitotic catastrophe. DNA Repair (Amst)
113.
Anantha, R. W., and Borowiec, J. A. (2009) Mitotic crisis: The unmasking of a
novel role for RPA. Cell Cycle 8, 12903-12908
114.
Shi, W., Feng, Z., Zhang, J., Gonzalez-Suarez, I., Vanderwaal, R. P., Wu, X.,
Powell, S. N., Roti Roti, J. L., and Gonzalo, S. (2010) The role of RPA2
phosphorylation in homologous recombination in response to replication arrest.
Carcinogenesis 31, 994-1002
115.
Liaw, H., Lee, D., and Myung, K. (2011) DNA-PK-dependent RPA2
hyperphosphorylation facilitates DNA repair and suppresses sister chromatid
exchange. PLoS ONE 6, e21424
116.
Binz, S. K., Lao, Y., Lowry, D. F., and Wold, M. S. (2003) The Phosphorylation
Domain of the 32-kDa Subunit of Replication Protein A (RPA) Modulates RPADNA Interactions: Evidence for an intersubunit interaction. J Biol Chem 278,
35584-35591
117.
Oakley, G. G., Patrick, S. M., Yao, J., Carty, M. P., Turchi, J. J., and Dixon, K.
(2003) RPA phosphorylation in mitosis alters DNA binding and protein-protein
interactions. Biochemistry 42, 3255-3264
118.
Liu, Y., Kvaratskhelia, M., Hess, S., Qu, Y., and Zou, Y. (2005) Modulation of
replication protein A function by its hyperphosphorylation-induced
195
conformational change involving DNA binding domain B. J Biol Chem 280,
32775-32783
119.
Dou, H., Huang, C., Singh, M., Carpenter, P. B., and Yeh, E. T. (2010)
Regulation of DNA Repair through DeSUMOylation and SUMOylation of
Replication Protein A Complex. Mol Cell 39, 333-345
120.
Hass, C. S., Lam, K., and Wold, M. S. (2012) Repair-specific Functions of
Replication Protein A. J Biol Chem 287, 3908-3918
121.
Brill, S. J., and Bastin-Shanower, S. (1998) Identification and characterization of
the fourth single-stranded-DNA binding domain of replication protein A. Mol
Cell Biol 18, 7225-7234
122.
Gibb, B., Ye, L. F., Gergoudis, S. C., Kwon, Y., Niu, H., Sung, P., and Greene, E.
C. (2014) Concentration-dependent exchange of replication protein a on singlestranded DNA revealed by single-molecule imaging. PLoS One 9, e87922
123.
Collins, B. E., Ye, L. F., Duzdevich, D., and Greene, E. C. (2014) DNA curtains:
novel tools for imaging protein-nucleic acid interactions at the single-molecule
level. Methods in cell biology 123, 217-234
124.
Chen, R., and Wold, M. S. (2014) Replication protein A: Single-stranded DNA's
first responder: Dynamic DNA-interactions allow replication protein A to direct
single-strand DNA intermediates into different pathways for synthesis or repair.
Bioessays 36, 1156-1161
125.
Kim, H. S., and Brill, S. J. (2001) Rfc4 interacts with Rpa1 and is required for
both DNA replication and DNA damage checkpoints in Saccharomyces
cerevisiae. Mol Cell Biol 21, 3725-3737
126.
Dodson, G. E., Shi, Y., and Tibbetts, R. S. (2004) DNA replication defects,
spontaneous DNA damage, and ATM-dependent checkpoint activation in
replication protein A-deficient cells. J Biol Chem 279, 34010-34014
127.
Zou, Y., Liu, Y., Wu, X., and Shell, S. M. (2006) Functions of human replication
protein A (RPA): From DNA replication to DNA damage and stress responses. J
Cell Physiol 208, 267-273
128.
Sugitani, N., and Chazin, W. J. (2014) Characteristics and concepts of dynamic
hub proteins in DNA processing machinery from studies of RPA. Progress in
biophysics and molecular biology
129.
Melendy, T., and Stillman, B. (1993) An interaction between replication protein A
and SV40 T antigen appears essential for primosome assembly during SV40 DNA
replication. J.Biol.Chem. 268, 3389-3395
196
130.
Yuzhakov, A., Kelman, Z., and O'Donnell, M. (1999) Trading places on DNA-a
three-point switch underlies primer handoff from primase to the replicative DNA
polymerase. Cell 96, 153-163
131.
Yeeles, J. T., Deegan, T. D., Janska, A., Early, A., and Diffley, J. F. (2015)
Regulated eukaryotic DNA replication origin firing with purified proteins. Nature
519, 431-435
132.
Iyama, T., and Wilson, D. M., 3rd. (2013) DNA repair mechanisms in dividing
and non-dividing cells. DNA Repair (Amst) 12, 620-636
133.
Genschel, J., and Modrich, P. (2009) Functions of MutL{alpha}, Replication
Protein A (RPA), and HMGB1 in 5'-Directed Mismatch Repair. J Biol Chem 284,
21536-21544
134.
Li, G. M. (2008) Mechanisms and functions of DNA mismatch repair. Cell
research 18, 85-98
135.
Riedl, T., Hanaoka, F., and Egly, J. M. (2003) The comings and goings of
nucleotide excision repair factors on damaged DNA. Embo J 22, 5293-5303
136.
You, J. S., Wang, M., and Lee, S. H. (2003) Biochemical analysis of the damage
recognition process in nucleotide excision repair. J Biol Chem 278, 7476-7485
137.
Walther, A. P., Gomes, X. V., Lao, Y., Lee, C. G., and Wold, M. S. (1999)
Replication protein A interactions with DNA. 1. Functions of the DNA-binding
and zinc-finger domains of the 70-kDa subunit. Biochemistry 38, 3963-3973
138.
Pestryakov, P. E., Khlimankov, D. Y., Bochkareva, E., Bochkarev, A., and
Lavrik, O. I. (2004) Human replication protein A (RPA) binds a primer-template
junction in the absence of its major ssDNA-binding domains. Nucleic Acids Res
32, 1894-1903
139.
Nguyen, B., Sokoloski, J., Galletto, R., Elson, E. L., Wold, M. S., and Lohman, T.
M. (2014) Diffusion of human replication protein A along single-stranded DNA. J
Mol Biol 426, 3246-3261
140.
Bastin-Shanower, S. A., and Brill, S. J. (2001) Functional analysis of the four
DNA binding domains of replication protein A - The role of RPA2 in ssDNA
binding. J Biol Chem, 36446-36453
141.
Beckett, D., Kovaleva, E., and Schatz, P. J. (1999) A minimal peptide substrate in
biotin holoenzyme synthetase-catalyzed biotinylation. Protein Sci 8, 921-929
142.
Henricksen, L. A., Umbricht, C. B., and Wold, M. S. (1994) Recombinant
replication protein A: Expression, complex formation, and functional
characterization. J Biol Chem 269, 11121-11132
197
143.
Binz, S. K., Dickson, A. M., Haring, S. J., and Wold, M. S. (2006) Functional
assays for replication protein A (RPA). Methods Enzymol 409, 11-38
144.
Joo, C., and Ha, T. (2012) Preparing sample chambers for single-molecule FRET.
Cold Spring Harbor protocols 2012, 1104-1108
145.
Honda, M., Park, J., Pugh, R. A., Ha, T., and Spies, M. (2009) Single-molecule
analysis reveals differential effect of ssDNA-binding proteins on DNA
translocation by XPD helicase. Mol Cell 35, 694-703
146.
Masuda-Ozawa, T., Hoang, T., Seo, Y. S., Chen, L. F., and Spies, M. (2013)
Single-molecule sorting reveals how ubiquitylation affects substrate recognition
and activities of FBH1 helicase. Nucleic Acids Res 41, 3576-3587
147.
Masuda-Ozawa, T., Hoang, T., Seo, Y.-S., Chen, L.-F., and Spies, M. (2013)
Single-molecule sorting reveals how ubiquitylation affects substrate recognition
and activities of FBH1 helicase. Nucleic Acids Res 41, in press
148.
van Oijen, A. M. (2011) Single-molecule approaches to characterizing kinetics of
biomolecular interactions. Current opinion in biotechnology 22, 75-80
149.
Bochkareva, E., Belegu, V., Korolev, S., and Bochkarev, A. (2001) Structure of
the major single-stranded DNA-binding domain of replication protein A suggests
a dynamic mechanism for DNA binding. EMBO J 20, 612-618
150.
Cai, L., Roginskaya, M., Qu, Y., Yang, Z., Xu, Y., and Zou, Y. (2007) Structural
characterization of human RPA sequential binding to single-stranded DNA using
ssDNA as a molecular ruler. Biochemistry 46, 8226-8233
151.
Nguyen, B., Sokoloski, J., Galletto, R., Elson, E., Wold, M. S., and Lohman, T.
M. (2014) Diffusion of human Replication Protein A along single stranded DNA.
Journal of Molecular Biology
152.
Lavrik, O. I., Kolpashchikov, D. M., Weisshart, K., Nasheuer, H. P., Khodyreva,
S. N., and Favre, A. (1999) RPA subunit arrangement near the 3'-end of the
primer is modulated by the length of the template strand and cooperative protein
interactions. Nucleic Acids Res. 27, 4235-4240
153.
Witosch, J., Wolf, E., and Mizuno, N. (2014) Architecture and ssDNA interaction
of the Timeless-Tipin-RPA complex. Nucleic acids research 42, 12912-12927
154.
De Laat, W. L., Appeldoorn, E., Sugasawa, K., Weterings, E., Jaspers, N. G. J.,
and Hoeijmakers, J. H. J. (1998) DNA-binding polarity of human replication
protein A positions nucleases in nucleotide excision repair. Genes Dev. 12, 25982609
198
155.
Shi, X., Jung, Y., Lin, L. J., Liu, C., Wu, C., Cann, I. K., and Ha, T. (2012)
Quantitative fluorescence labeling of aldehyde-tagged proteins for singlemolecule imaging. Nat Methods
156.
Pretto, D. I., Tsutakawa, S., Brosey, C. A., Castillo, A., Chagot, M. E., Smith, J.
A., Tainer, J. A., and Chazin, W. J. (2010) Structural dynamics and ssDNA
binding activity of the three N-terminal domains of the large subunit of
Replication Protein A from small angle X-ray scattering. Biochemistry
157.
Murphy, M. C., Rasnik, I., Cheng, W., Lohman, T. M., and Ha, T. (2004) Probing
single-stranded DNA conformational flexibility using fluorescence spectroscopy.
Biophys J 86, 2530-2537
158.
Jacobs, D. M., Lipton, A. S., Isern, N. G., Daughdrill, G. W., Lowry, D. F.,
Gomes, X., and Wold, M. S. (1999) Human replication protein A: Global fold of
the N-terminal RPA-70 domain reveals a basic cleft and flexible C-terminal
linker. J Biomol NMR 14, 321-331
159.
Buchko, G. W., Daughdrill, G. W., De Lorimier, R., Rao, S., Isern, N. G.,
Lingbeck, J. M., Taylor, J. S., Wold, M. S., Gochin, M., Spicer, L. D., Lowry, D.
F., and Kennedy, M. A. (1999) Interactions of human nucleotide excision repair
protein XPA with DNA and RPA70DeltaC327: Chemical shift mapping and 15N
NMR relaxation studies. Biochemistry 38, 15116-15128
160.
Daughdrill, G. W., Buchko, G. W., Botuyan, M. V., Arrowsmith, C., Wold, M. S.,
Kennedy, M. A., and Lowry, D. F. (2003) Chemical shift changes provide
evidence for overlapping single-stranded DNA- and XPA-binding sites on the 70
kDa subunit of human replication protein A. Nucleic Acids Res 31, 4176-4183
161.
Brosey, C. A., Soss, S. E., Brooks, S., Yan, C., Ivanov, I., Dorai, K., and Chazin,
W. J. (2015) Functional Dynamics in Replication Protein A DNA Binding and
Protein Recruitment Domains. Structure
162.
Overmeer, R. M., Moser, J., Volker, M., Kool, H., Tomkinson, A. E., van
Zeeland, A. A., Mullenders, L. H., and Fousteri, M. (2011) Replication protein A
safeguards genome integrity by controlling NER incision events. J Cell Biol
163.
Deng, S. K., Chen, H., and Symington, L. S. (2014) Replication protein A
prevents promiscuous annealing between short sequence homologies:
Implications for genome integrity. Bioessays
164.
Bhat, K. P., Betous, R., and Cortez, D. (2015) High-affinity DNA-binding
domains of replication protein A (RPA) direct SMARCAL1-dependent replication
fork remodeling. J Biol Chem 290, 4110-4117
165.
Burhans, W. C., and Weinberger, M. (2007) DNA replication stress, genome
instability and aging. Nucleic Acids Res 35, 7545-7556
199
166.
Bochkareva, E., Frappier, L., Edwards, A. M., and Bochkarev, A. (1998) The
RPA32 subunit of human replication protein A contains a single-stranded DNAbinding domain. J Biol Chem 273, 3932-3936
167.
Patrick, S. M., and Turchi, J. J. (2001) Stopped-flow kinetic analysis of
replication protein A-binding DNA - Damage recognition and affinity for singlestranded DNA reveal differential contributions of k(on) and k(off) rate constants.
Journal of Biological Chemistry 276, 22630-22637
168.
Atrazhev, A., Zhang, S., and Grosse, F. (1992) Single-stranded DNA binding
protein from calf thymus--Purification, properties, and stimulation of the
homologous DNA-polymerase-a-primase complex. Eur.J.Biochem. 210, 855-865
169.
Nakagawa, M., Tsukada, S., Soma, T., Shimuzu, Y., Miyake, S., Iwamatsu, A.,
and Sugiyama, H. (1991) cDNA cloning of the murine 30-kDa protein
homologous to the 32-kDa subunit of human replication protein A. Nucleic Acids
Res. 19, 4292
170.
Adachi, Y., and Laemmli, U. K. (1992) Identification of nuclear pre-replication
centers poised for DNA synthesis in Xenopus egg extracts: Immunolocalization
study of replication protein A. J.Cell Biol. 119, 1-15
171.
Brill, S. J., and Stillman, B. (1989) Yeast replication factor-A functions in the
unwinding of the SV40 origin of DNA replication. Nature 342, 92-95
172.
Brown, G. W., and Ray, D. S. (1992) Purification and characterization of DNA
ligase I from the trypanosomatid Crithidia fasciculata. Nucleic Acids Res. 20,
3905-3910
173.
Millership, J. J., and Zhu, G. (2002) Heterogeneous expression and functional
analysis of two distinct replication protein A large subunits from Cryptosporidium
parvum. Int J Parasitol 32, 1477-1485
174.
Higgs, H. N. (2005) Formin proteins: a domain-based approach. Trends Biochem
Sci 30, 342-353
175.
Tomkiel, J. E., Alansari, H., Tang, N., Virgin, J. B., Yang, X., VandeVord, P.,
Karvonen, R. L., Granda, J. L., Kraut, M. J., Ensley, J. F., and Fernandez-Madrid,
F. (2002) Autoimmunity to the M(r) 32,000 subunit of replication protein A in
breast cancer. Clin Cancer Res 8, 752-758
176.
Haring, S. J., Humphreys, T. D., and Wold, M. S. (2009) A naturally occurring
human RPA subunit homolog does not support DNA replication or cell-cycle
progression. Nucleic Acids Res
177.
Mason, A. C., Roy, R., Simmons, D. T., and Wold, M. S. (2010) Functions of
alternative Replication Protein A (aRPA) in initiation and elongation.
Biochemistry
200
178.
Choi, J. H., Lindsey-Boltz, L. A., Kemp, M., Mason, A. C., Wold, M. S., and
Sancar, A. Reconstitution of RPA-covered single-stranded DNA-activated ATRChk1 signaling. Proceedings of the National Academy of Sciences of the United
States of America 107, 13660-13665
179.
De Laat, W. L., Jaspers, N. G. J., and Hoeijmakers, J. H. J. (1999) Molecular
mechanism of nucleotide excision repair. Genes Dev. 13, 768-785
180.
Sancar, A. (1996) DNA excision repair. Annu.Rev.Biochem. 65, 43-81
181.
Wood, R. D. (1997) Nucleotide excision repair in mammalian cells. J.Biol.Chem.
272, 23465-23468
182.
Reardon, J. T., and Sancar, A. (2003) Recognition and repair of the cyclobutane
thymine dimer, a major cause of skin cancers, by the human excision nuclease.
Genes Dev 17, 2539-2551
183.
Li, L., Lu, X. Y., Peterson, C. A., and Legerski, R. J. (1995) An interaction
between the DNA repair factor XPA and replication protein a appears essential
for nucleotide excision repair. Mol.Cell.Biol. 15, 5396-5402
184.
Matsunaga, T., Park, C. H., Bessho, T., Mu, D., and Sancar, A. (1996) Replication
protein A confers structure-specific endonuclease activities to the XPF-ERCC1
and XPG subunits of human DNA repair excision nucleases. J.Biol.Chem. 271,
11047-11050
185.
Li, X., and Heyer, W. D. (2008) Homologous recombination in DNA repair and
DNA damage tolerance. Cell research 18, 99-113
186.
Golub, E. I., Gupta, R. C., Haaf, T., Wold, M. S., and Radding, C. M. (1998)
Interaction of human Rad51 recombination protein with single-stranded DNA
binding protein, RPA. Nucleic Acids Res 26, 5388-5393
187.
Park, M. S., Ludwig, D. L., Stigger, E., and Lee, S. H. (1996) Physical interaction
between human RAD52 and RPA is required for homologous recombination in
mammalian cells. J Biol Chem 271, 18996-19000
188.
Sugiyama, T., Zaitseva, E. M., and Kowalczykowski, S. C. (1997) A singlestranded DNA-binding protein is needed for efficient presynaptic complex
formation by the Saccharomyces cerevisiae Rad51 protein. J.Biol.Chem. 272,
7940-7945
189.
Sung, P. (1997) Communication - Function of yeast Rad52 protein as a mediator
between replication protein A and the Rad51 recombinase. J.Biol.Chem. 272,
28194-28197
201
190.
New, J. H., Sugiyama, T., Zaitseva, E., and Kowalczykowski, S. C. (1998) Rad52
protein stimulates DNA strand exchange by Rad51 and replication protein A.
Nature 391, 407-410
191.
Givalos, N., Gakiopoulou, H., Skliri, M., Bousboukea, K., Konstantinidou, A. E.,
Korkolopoulou, P., Lelouda, M., Kouraklis, G., Patsouris, E., and Karatzas, G.
(2007) Replication protein A is an independent prognostic indicator with potential
therapeutic implications in colon cancer. Mod Pathol 20, 159-166
192.
Vassin, V. M., Wold, M. S., and Borowiec, J. A. (2004) Replication protein A
(RPA) phosphorylation prevents RPA association with replication centers. Mol
Cell Biol 24, 1930-1943
193.
Hass, C. S., Lam, K., and Wold, M. S. (2011) Repair-specific functions of
Replication Protein A. J Biol Chem
194.
Jiang, K., Pereira, E., Maxfield, M., Russell, B., Goudelock, D. M., and Sanchez,
Y. (2003) Regulation of Chk1 includes chromatin association and 14-3-3 binding
following phosphorylation on Ser-345. J Biol Chem 278, 25207-25217
195.
Kuo, L. J., and Yang, L. X. (2008) Gamma-H2AX - a novel biomarker for DNA
double-strand breaks. In Vivo 22, 305-309
196.
Sharma, A., Singh, K., and Almasan, A. (2012) Histone H2AX phosphorylation: a
marker for DNA damage. Methods Mol Biol 920, 613-626
197.
Li, Z., Musich, P. R., Serrano, M. A., Dong, Z., and Zou, Y. (2011) XPAmediated regulation of global nucleotide excision repair by ATR Is p53dependent and occurs primarily in S-phase. PloS one 6, e28326
198.
Humphries, A., and Wright, N. A. (2008) Colonic crypt organization and
tumorigenesis. Nat Rev Cancer 8, 415-424
199.
Kim, K. M., and Shibata, D. (2002) Methylation reveals a niche: stem cell
succession in human colon crypts. Oncogene 21, 5441-5449
200.
Lopez Castel, A., Nakamori, M., Tome, S., Chitayat, D., Gourdon, G., Thornton,
C. A., and Pearson, C. E. (2011) Expanded CTG repeat demarcates a boundary
for abnormal CpG methylation in myotonic dystrophy patient tissues. Hum Mol
Genet 20, 1-15
201.
Lopez Castel, A., Cleary, J. D., and Pearson, C. E. (2010) Repeat instability as the
basis for human diseases and as a potential target for therapy. Nature reviews 11,
165-170
202.
Seriola, A., Spits, C., Simard, J. P., Hilven, P., Haentjens, P., Pearson, C. E., and
Sermon, K. (2011) Huntington's and myotonic dystrophy hESCs: down-regulated
202
trinucleotide repeat instability and mismatch repair machinery expression upon
differentiation. Hum Mol Genet 20, 176-185
203.
Pearson, C. E., Nichol Edamura, K., and Cleary, J. D. (2005) Repeat instability:
mechanisms of dynamic mutations. Nat Rev Genet 6, 729-742
204.
Mirkin, S. M. (2007) Expandable DNA repeats and human disease. Nature 447,
932-940
205.
Slean, M. M., Panigrahi, G. B., Ranum, L. P., and Pearson, C. E. (2008)
Mutagenic roles of DNA "repair" proteins in antibody diversity and diseaseassociated trinucleotide repeat instability. DNA Repair (Amst) 7, 1135-1154
206.
Tome, S., Manley, K., Simard, J. P., Clark, G. W., Slean, M. M., Swami, M.,
Shelbourne, P. F., Tillier, E. R., Monckton, D. G., Messer, A., and Pearson, C. E.
(2013) MSH3 polymorphisms and protein levels affect CAG repeat instability in
Huntington's disease mice. PLoS Genet 9, e1003280
207.
Panigrahi, G. B., Slean, M. M., Simard, J. P., Gileadi, O., and Pearson, C. E.
(2010) Isolated short CTG/CAG DNA slip-outs are repaired efficiently by
hMutSbeta, but clustered slip-outs are poorly repaired. Proc Natl Acad Sci U S A
107, 12593-12598
208.
Hou, C., Chan, N. L., Gu, L., and Li, G. M. (2009) Incision-dependent and errorfree repair of (CAG)(n)/(CTG)(n) hairpins in human cell extracts. Nat Struct Mol
Biol 16, 869-875
209.
Panigrahi, G. B., Lau, R., Montgomery, S. E., Leonard, M. R., and Pearson, C. E.
(2005) Slipped (CTG)*(CAG) repeats can be correctly repaired, escape repair or
undergo error-prone repair. Nat Struct Mol Biol 12, 654-662
210.
Stuchbury, G., and Munch, G. (2010) Optimizing the generation of stable
neuronal cell lines via pre-transfection restriction enzyme digestion of plasmid
DNA. Cytotechnology 62, 189-194
211.
Vassin, V. M., Anantha, R. W., Sokolova, E., Kanner, S., and Borowiec, J. A.
(2009) Human RPA phosphorylation by ATR stimulates DNA synthesis and
prevents ssDNA accumulation during DNA-replication stress. J Cell Sci 122,
4070-4080
212.
Din, S., Brill, S. J., Fairman, M. P., and Stillman, B. (1990) Cell-cycle-regulated
phosphorylation of DNA replication factor A from human and yeast cells. Genes
& development 4, 968-977
213.
Shao, R. G., Cao, C. X., Zhang, H., Kohn, K. W., Wold, M. S., and Pommier, Y.
(1999) Replication-mediated DNA damage by camptothecin induces
phosphorylation of RPA by DNA-dependent protein kinase and dissociates
RPA:DNA-PK complexes. Embo J 18, 1397-1406
203
214.
Block, W. D., Yu, Y., and Lees-Miller, S. P. (2004) Phosphatidyl inositol 3kinase-like serine/threonine protein kinases (PIKKs) are required for DNA
damage-induced phosphorylation of the 32 kDa subunit of replication protein A at
threonine 21. Nucleic acids research 32, 997-1005
215.
Oakley, G. G., Loberg, L. I., Yao, J., Risinger, M. A., Yunker, R. L., ZernikKobak, M., Khanna, K. K., Lavin, M. F., Carty, M. P., and Dixon, K. (2001) UVinduced hyperphosphorylation of replication protein a depends on DNA
replication and expression of ATM protein. Molecular biology of the cell 12,
1199-1213
216.
Oakley, G. G., and Patrick, S. M. (2010) Replication protein A: directing traffic at
the intersection of replication and repair. Frontiers in bioscience 15, 883-900
217.
Dutta, A., and Stillman, B. (1992) cdc2 family kinases phosphorylate a human
cell DNA replication factor, RPA, and activate DNA replication. The EMBO
journal 11, 2189-2199
218.
Pan, Z. Q., Amin, A. A., Gibbs, E., Niu, H., and Hurwitz, J. (1994)
Phosphorylation of the p34 subunit of human single-stranded-DNA-binding
protein in cyclin A-activated G1 extracts is catalyzed by cdk-cyclin A complex
and DNA-dependent protein kinase. Proceedings of the National Academy of
Sciences of the United States of America 91, 8343-8347
219.
Fang, F., and Newport, J. W. (1993) Distinct roles of cdk2 and cdc2 in RP-A
phosphorylation during the cell cycle. Journal of cell science 106 ( Pt 3), 983-994
220.
Zernik-Kobak, M., Vasunia, K., Connelly, M., Anderson, C. W., and Dixon, K.
(1997) Sites of UV-induced phosphorylation of the p34 subunit of replication
protein A from HeLa cells. The Journal of biological chemistry 272, 23896-23904
221.
Anantha, R. W., Vassin, V. M., and Borowiec, J. A. (2007) Sequential and
Synergistic Modification of Human RPA Stimulates Chromosomal DNA Repair.
J Biol Chem 282, 35910-35923
222.
Binz, S. K., Lao, Y., Lowry, D. F., and Wold, M. S. (2003) The phosphorylation
domain of the 32-kDa subunit of replication protein A (RPA) modulates RPADNA interactions. Evidence for an intersubunit interaction. The Journal of
biological chemistry 278, 35584-35591
223.
Dornreiter, I., Erdile, L. F., Gilbert, I. U., von Winkler, D., Kelly, T. J., and
Fanning, E. (1992) Interaction of DNA polymerase alpha-primase with cellular
replication protein A and SV40 T antigen. The EMBO journal 11, 769-776
224.
Dutta, A., and Stillman, B. (1992) cdc2 Family kinases phosphorylate a human
cell DNA replication factor, RPA, and activate DNA replication. EMBO J. 11,
2189-2199
204
225.
Shi, W., Feng, Z., Zhang, J., Gonzalez-Suarez, I., Vanderwaal, R. P., Wu, X.,
Powell, S. N., Roti Roti, J. L., Gonzalo, S., and Zhang, J. (2010) The role of
RPA2 phosphorylation in homologous recombination in response to replication
arrest. Carcinogenesis 31, 994-1002
226.
Roy, R., Hohng, S., and Ha, T. (2008) A practical guide to single-molecule FRET.
Nat Methods 5, 507-516