* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Biological AFM Setup
Hedgehog signaling pathway wikipedia , lookup
Phosphorylation wikipedia , lookup
List of types of proteins wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Magnesium transporter wikipedia , lookup
Protein design wikipedia , lookup
Protein moonlighting wikipedia , lookup
Protein phosphorylation wikipedia , lookup
Protein structure prediction wikipedia , lookup
Protein folding wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
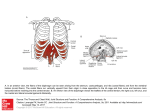
Protein Assemblies in Health and in Diseases: Biological AFM and Related Studies Havisha Garimella1,2 Preceptors: Dr. Albert J. Jin1, Dr. Paul Smith1 1Laboratory Abstract Interpretation Results Alzheimer's disease affects more than 5 million people; it cannot yet be cured[1]. Amyloid β protein is known to be associated with Alzheimer’s, as large deposits of it were found in the brain of patients with Alzheimer's. Thousands of monomers of the amyloid β build up exponentially in the brain, forming fibers. The problem lies in the fact that the pathogenicity of the amyloid β, or how it exactly causes Alzheimer’s, is unknown. There is a belief, though not as well known, that the true culprit is the small soluble oligomers that makeup the polymers, with protein conformational changes from random coil to alpha-helix, and to beta-sheets. The atomic force microscopy (AFM), which provides high resolution 3D visualization at the nanoscale [2], is used in the study. The machine is used to identify and record protein oligomers’ conformational changes and shape in vitro, at all stages of the polymerization of the amyloid β, that correlate to the loss of axon function in Alzheimer’s. The structural biology and mechanics of protein assembly of the amyloid β protein can be outlined through the AFM, which will give us insight into its pathogenicity. The study of the amyloidosis of amyloid β is central to the pathology of Alzheimer’s. It is necessary to understand on a macromolecular level what triggers the complex folding mechanisms and shifts the equilibrium from functional to pathological isoforms of proteins. By doing so we can map out the pathway of amyloid β and inhibit the protein assembly. Healthy Brain of Cellular Imaging and Macromolecular Biophysics, National Institute of Biomedical Imaging and Bioengineering, NIH, Bethesda, MD, USA; 2Mount Hebron High School, Maryland, USA. AFM Characterization The reason why the APS modified surface worked, as opposed to the Ca2+ modified surface, is because the APS surface is much smoother. Ca has a tendency to crystallize and therefore does not produce as uniform of a surface. Data and Analysis No growth without NaCl 5 nm 2 hr on APS mica. Can see that there are more fibers and they have grown longer Conclusion 0 nm Advanced Alzheimer’s 40 min on APS mica. Can see the fibers beginning to form More salt enables proteins to overcome charge repulsion and so they come together. There is a significant change in height from the 40 minutes to the 2 hours. This could be because initially, when the fiber is forming, there are a lot of nonstructural aggregates, i.e. salt, forming around it, so it causes the height to be larger than normal. After the monomers build up and fibers form, the aggregates disperse, giving a more accurate measurement of the height of the protein itself. There are so many short pieces of fibers in the solution because when the aggregate disperses, some of the protein comes with it. Because there is less salt aggregate, the fibers are not as thick as it was in the initial stages; however, the thickness does increase when fibers get tangled (7.4 nm). Also, there is a significant increase in length. It is believed that the oligomers do no stack on top of each other, but instead build up next to each other because the length gets larger, but not the height. 100 nm 70 hr on APS mica. Can see that the fibers are much longer and associating with one another possibly forming plaques. Left. Healthy brain compared to brain affected by Alzheimer's[3]. Above. (Shown above.) Diagram of AFM setup [2]. Materials & Methods Biological AFM Setup The MultiMode AFM was used. Setup was as follows: 1. Prepared mica layer on top of silicon disc for Multimode AFM. Peeled layer off using Scotch tape for a fully flat surface. 2. Aligned laser onto cantilever and engaged tip onto surface. 3. Set to tapping mode for readout. 4. Executed high-resolution scans. 5. Analyzed data with Nanoscope software and ImageJ. Above. MultiMode AFM (Bruker, CA). Parameters for AFM Characterization 1mg/mL of Aβ (1-40aa) in 40 mM Tris-HC, pH 8.0, with 100 mM NaCl at 25o C was imaged • Protein taken from lyophilized Aβ and added to the salt solution, at which point the assembly process begins. • At various time points 5 µL of the assembling fibers solution is taken out and diluted 100-fold into no salt 40mM, Tris-HC, pH 8.0, to stop the assembly. Doing so allows us to look at the various time points of fiber formation under the AFM, without worrying that the fibers are still assembling. • Samples imaged on mica surface of; aminopropyl silatrane (APS). The modification gives the surface + charge (see diagram). This was because it was thought that the fibers were negatively charged because the calculated isoelectric point of the Aβ was 6. Studying this protein is a necessity because experts are certain that amyloid β causes Alzheimer’s and other neurodegenerative diseases as well. The key part of this study is to first characterize the protein assembly of amyloid β. There is very little information regarding the structure and kinetics of the Aβ, thus before one can understand the protein’s relationship with Alzheimer’s, it is necessary to outline the fiber formations of the protein. From the data and analysis, it is concluded that the Aβ protein elongates. It initially aggregates a lot with the salt and biological media, but later as the fibers form, the nonstructural aggregates disperse. Also, the protein oligomers stack up next to each other. Moreover, another vital discovery is that more salt enables this protein to grow. Significant progress has been made as there are very few studies published on the structural analysis of this protein; however, through this experiment, we were able to provide biophysical characterization of it. This is very important because by understanding the structure, we will be able to map out the pathway, learn what specific oligomers correlate to axon loss, and inhibit the protein assembly. The initial steps of the project have been completed, which is to grow the fibers and record and study conformational changes at all stages of the polymerization of amyloid β. Now the future work required is to integrate dynamic light scattering, mathematical modeling, optical Imaging, raman and fluorescence spectroscopy, and theoretical analysis to identify its pathogenicity, to learn how to prevent the protein growth, and to simply enhance our information regarding its macromolecular pathway. The study of the amyloidosis of amyloid β is central to the pathology of Alzheimer’s.