* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Modulation of Gene Expression by Scaffold/Matrix Attached Regions
Survey
Document related concepts
Transcript
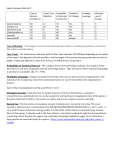
1 Transcriptional Augmentation: Modulation of Gene Expression by Scaffold/Matrix Attached Regions (S/MAR Elements) Jürgen Bode1, Craig Benham2, Angela Knopp1 and Christian Mielke3 1 GBF, National Center for Biotechnological Research, D-38124 Braunschweig, Mascheroder Weg 1 2 Department of Biomathematical Sciences, Box 1023, Mount Sinai School of Medicine, 1 Gustave Levy Place, New York, NY 10029 3 University of Aarhus, Dept. of Molec. and Struct. Biology, C.F. Møllers Alle 131, DK-8000 Aarhus C Denmark ABSTRACT: For a long time S/MARs could only be characterized by the assays in vitro that led to their detection.. Only recently a number of biological activities emerged which are common to most or all S/MARs that are detected by the classical procedures. This review will focus on the phenomenon of transcriptional augmentation which is found for genomically anchored or episomal genes and on a group of partially overlapping activities which are suited to maintain an episomal status. It is further attempted to correlate properties of the S/MAR-scaffold interaction with prominent or prototype protein binding partners. Keywords: base-unpairing region (BUR), chromatin domains, episomal vectors, scaffold/matrix attached regions, stress-induced duplex destabilization (SIDD), transcriptional augmentation, unwinding elements (UE). I Introduction: Biological Activities associated with S/MARs The proteinaceous intranuclear framework, called either `nuclear matrixA (Berezney and Coffey, 1974) or `nuclear scaffoldA (Mirkovitch et al., 1984), is thought to mediate the domain organization of the eukaryotic nucleus. Branched core filaments provide a supporting structure for the formation of DNA loops and participate in diverse matrix-supported processes such as DNA replication, -transcription and -recombination, RNA-processing and -transport as well as signal transduction and apoptotic events (review: Berezney et al., 1995). The DNA elements which mediate the attachment of chromatin loops, so called scaffold/matrix attached regions (S/MARs), have attracted considerable interest due a number of rather distinct structure-function relationships. S/MARs of several kilobasepairs are found at the borders of chromatin domains, and shorter elements with basically the same physicochemical properties occur in close association with certain enhancers or in introns. Accordingly, S/MARs are found either in nontranscribed regions or within transcription units, but rarely if ever in coding regions. A wide range of activities has been ascribed to S/MARs, among these an insulator function whereby two of these elements, bracketing a transcription unit, uncouple the gene from chromosome position effects (reviewed in Bode et al., 1998) and a function as recombination hotspots which involves nuclear matrix functions (Strissel et al., 1998). Our review will concentrate 2 on two aspects which have already received wide acceptance: the transcriptional (`augmentingA) activity of S/MARs and their apparent function(s) in episomes. In addition we will discuss some characterized protein binding partners and their possible contribution to these effects. II Characteristics of S/MAR-Scaffold Recognition S/MARs have been operationally defined according to the protocols that lead to their detection. There are two basic criteria: first, S/MARs constitute those endogenous DNA fragments that co-purify with the nuclear matrix (i.e. remain bound to the nuclear matrix after chromatin proteins and DNA in the chromatin loops have been removed) or second, S/MARs represent those exogenously added DNA fragments that bind to a purified nuclear scaffold in the presence of (prokaryotic) competitor DNA. While the first (halo mapping) approach may reveal some features of the scaffold/matrix interaction in the cell (Bode et al., 1996), the latter (re-association) approach is well suited as a means by which the physicochemical properties of the interaction can be quantified. An obvious advantage of the reassociation methodology is the fact that affinity parameters can be simulated by computation with the final aim to localize and classify S/MARs directly from sequence information. It is anticipated that these concepts can be successfully applied to study the domain organization of higher cells in the framework of ongoing eukaryotic genome projects. Fig. 1 underlines the complexity of recogniton features for a nuclear substructure, called `scaffoldA, that is obtained after the extraction of cell nuclei with lithium 3,5-diiodosalicylate (LIS). The initially supercoiled (`scA) S/MAR vector is firmly bound to the scaffold until persistent topoisomerase activities lower its superhelical density from σ = -0.055 to - 0.040 (Kay and Bode 1995). Concomitant with topoisomerization a nicking activity in the scaffold generates a relaxed circle (`nickedA). This circle is partially bound, but only in case a S/MAR sequence is present. S/MAR recognition by the scaffold becomes optimal only after this nicked circle has further been processed to a linear fragment (`linA), i.e. under the conditions of the standard reassociation assay. These observations indicate that an efficient S/MAR interaction requires a considerable amount of conformational flexibility or exposure of single strands due to superhelical strain. III Anatomy of a S/MAR: UEs, CUEs and the BUR Chemical probing studies by single-strand specific enzymes or reagents have shown that S/MARs can undergo strand separation in vivo (reviewed in Bode et al., 1996) or under the negative superhelicity of a plasmid in vitro (Bode et al., 1992). Interestingly, these reactivity data can be matched by computer analysis after applying superhelical tension (Benham et al., 1997 and Fig. 2). For these analyses the sequences in question are analyzed as part of a plasmid, i.e. under the superhelical density as it exists for the in vitro experiment (σ = -0.055). Although prokaryotes do not contain S/MAR-type sequences (which is most evident from the fact that prokaryotic DNA at any excess does not interfere with a S/MAR-scaffold re-association in vitro) common bacterial 3 plasmids show two well characterized, narrow unwinding elements (UEs) that flank the ampicillin resistance gene (Benham, 1997). Although these sites are too restricted to mediate scaffold attachment, they serve as convenient internal standards for the quantification of the related, but more extensive features in prototype S/MARs. If we clone a 2.2 kb S/MAR sequence, derived from the human interferon-ß upstream domain border, into our standard pTZ18R vector, it forms an extended base-unpairing region (BUR). This BUR consists of multiple UEs some of which compete efficiently with the mentioned internal markers. Among the UEs there may be a prominent one, the so called `core-unwinding elementA (CUE) and this is usually the nucleation center of unwinding which is preferentially modified by single-strand specific reagents such as chloroacetaldehyde or potassium permanganate (see the signal at the CUE-position in Fig. 2). The S/MAR insert, called `IA in Mielke et al. (1990), and an 800 bp subfragment (`IVA, ibid.) which include the CUE are prototype elements that have been used by us and others in a variety of biological test systems. Where applicable, we will refer to these elements below. During the analysis of multiple S/MARs both from animal and plant sources we have observed a tight correlation between the scaffold association strength and the following parameters: i - length of the BUR, ii - number, extension and extent of individual destabilized sites and iii - the spacing between the UEs (Bode et al., in preparation). Individual destabilized UEs may correspond to the `AT-patchesA described by Tsutsui (1998 ) or the `90% AT boxA observed by Michalowski et al. (1999). While many S/MARs are AT rich per se, this is not a stringent criterion since an even distribution of AT-patches, separated by short GC-rich spacers, is also able to confer binding affinity. IV Transcriptional Augmentation S/MARs have been shown to stimulate transcription initiation rates (Schübeler et al., 1996) in a wide variety of cell lines and sometimes also in transgenic organisms suggesting a rather widespread transcriptional facilitator function. This action is particularly evident if an integrated heterologous reporter gene is flanked by two S/MARs thereby forming a `minidomainA. Increased transcription is largely independent of the source of the elements, it parallels their binding strength to a LIS-scaffold (Kay and Bode 1995) and it is also seen for artificial constructs which have been obtained by oligomerizing a CUE up to the critical minimum extension. These findings lend support to the idea that S/MAR functions are not strictly dependent on sequence but rather on secondary structure(s). A S/MAR-stimulated expression (`augmentationA) is never observed in transient assays but it requires stable integration into the genome suggesting that S/MARs exert their effect by modulating chromatin structures (Poljak et al., 1994; reviews Bode et al., 1995, 1996, 1998). Fig. 3 demonstrates these basic facts and also indicates the importance of distance between the promoter and the S/MAR element. For this particular experiment we have used a retroviral gene 4 transfer system which integrates a single, intact copy (the so-called provirus) into a subclass of genomic sites. The provirus contains a long transcription unit formed by a bicistronic construct and allows cloning of an 800 bp minimal S/MAR (element `IVA) at various distances (positions a-c, 3´ and 5´3´) from the promoter which resides in the 5´ long terminal repeat (LTR). The lefthand diagram demonstrates that all constructs are functional since they are transcribed in the transient phase of gene expression. Here, transcriptional initiation is maximal for the S/MAR free control (Sp) but is inhibited (20-60%) in the presence of the S/MAR. Expression is monitored by mRNA levels as well as by the reporter genes yielding consistent data (except for SEAP expression in construct Sp-a which is explained by multiple cryptic translation-initiation sites in the upstream S/MAR; Schübeler et al., 1996). These relationships are dramatically changed upon integration of the transgene, i.e. its covalent anchoring to the host cell genome. Now transcriptional initiation rates are up-regulated by the distal S/MAR (in Sp-c) and for the `minidomainA situation (i.e. the 5´-S/MAR-gene-3´-S/MAR arrangement seen in Sp-5´3´). This minidomain arises as a consequence of the retroviral integration mechanism during which the complete sequence information of the 3´LTR is copied and transferred to the 5´-LTR. It is noted that this `double copy vectorA principle has been successfully applied for the construction of high and stably expressing therapeutic retroviral vectors using fragment `IVA (Auten et al., 1999). Transcriptional augmentation, i.e. the increase of initiation rates in the integrated but not in the pre-integrative state, is the most stringent criterion to discriminate S/MAR and enhancer effects since a prototype enhancement would occur in both states. The inefficiency of S/MARs prior to integration might be a consequence of the fact that transiently transfected genes are transcribed with ease since they adopt an open if any chromatin structure. While enhancers would add efficiency by stabilizing the transcription initiation complex, an element overcoming restrictions due to an ordered chromatin structure appears to be unnecessary. A status with characteristics both of a transiently expressed template and an integrated copy is the replicating episome. We will devote a separate chapter to the function of S/MARs in episomes which appear to utilize a highly specific set of interactions for the support of transcription and for replication functions (chapter IV.B.6). A detailed inspection of Fig. 3 demonstrates that the augmenting function of a S/MAR critically depends on its distance from the promoter and the direction of transcription. This phenomenon is explained best by considering the topological changes of the DNA template occurring during transcription and the potential role S/MARs may play in relieving superhelical strain (chapter IVB1). A Transcriptional competence The hallmark of complex organisms is differential, tissue-specific gene expression. Although the states of expression are stable and can be propagated through many cell divisions, 5 they do not generally reflect genetic differences but rather epigenetic mechanisms that rely on DNA sequence patterns contained in nuclear non-coding DNA. Special sequences with regulatory potential such as introns, locus-control regions (LCRs), S/MARs and repetitive elements are found in these regions. Thus, the genome can be considered a mosaic of sequence motifs which cooperate to determine the state of an organism. Four successive events, potentiation, initiation, elongation and termination have been defined which represent an ordered process for balanced transcription (review Krawetz et al., 1999). Potentiation creates transcriptional competence, for instance by changing the local chromatin conformation from a compact 30 nm fiber to the open 10 nm fiber. This change is a prerequisite for gene expression as it permits the action of transcription factors that are specific for the respective gene domain. There are models which implicate S/MARs in establishing transcriptional competence and others considering their immediate influence on the transcriptional level. We will use these principal modes of action to structure this paragraph well knowing that some phenomena may fall in between these two phases .1 Domain size While it is generally agreed that the average size of a chromatin domain in a eukaryotic cell is around 70 kb, the natural distribution of S/MARs reveals sizes ranging between 3 and about 200 kb (Gasser and Laemmli, 1987). Generally the smaller loop sizes are assigned to genes that can be highly transcribed under certain circumstances and prototype examples for this may be the histone gene cluster (5 kb) which is regulated in a cell-cycle dependent fashion and the type I interferon gene cluster (loop sizes 3-14 kb; Strissel et al., 1998) members of which are rapidly activated following a viral infection. It is proposed that these loci are permanently potentiated as a possible consequence of the close apposition of S/MARs. S/MARs repeated over a short distance might sterically interfere with a cooperative 10 to 30 nm fiber transition and thereby counteract inactivation. In accord with such a model an artificial S/MAR-luciferase-S/MAR minidomain with a 3 kb loop was found to remain active after transfection for more than 3 month whereas a truncated control (S/MAR-luciferase) construct, for which the loop size is determined by the genomic site of integration, lost half its expression over a period of 6 weeks (Bode et al., 1995). In contrast to these small, permanently open domains, genes that are only expressed in distinct cell types or at certain stages of development are typically embedded in larger domains which have to acquire transcriptional competence under the respective circumstances. 2 Displacement of histone H1 by D-proteins: The active Mechanism Are S/MARs involved in active domain opening processes? The specificity of the S/MARscaffold interaction is mediated by proteins recognizing certain non-B structural features. One of the more prominent components co-purifying with the scaffold is histone H1 (Tab. 1) which plays a central role in the compaction of chromatin into a transcriptionally silent fiber. Nucleation of H1 6 assembly has been shown to occur on S/MARs, especially on An tracts, from where this histone spreads in a cooperative manner to occupy flanking DNA. These A-tracts can be titrated with distamycin whereby the cooperative interactions are broken, H1 is redistributed and transcription from the S/MAR templates becomes de-repressed. An extensive search for proteins with a Distamycin-like specificity led to a `D-proteinA candidate, HMG-I/Y, which is known to bind to Atracts longer than four AT base pairs. HMG I/Y binding in turn may be facilitated by HMG-1 which recognizes deformed or deformable DNA without any defined sequence specificity (Käs et al., 1993). Thompson et al. (1994) have generated transgenic mice homozygous for a heat shock gene which is transcribed already at the two-cell stage. During development the same gene is expressed in a number of differentiated tissues. Transgenic lines were generated from constructs flanked by S/MAR element `IA and the respective downstream border from the human interferon ß domain or from S/MAR-free controls. Prior Before the onset of differentiation S/MARs augmented transgene expression in a consistent, position-independent manner. In contrast, in the differentiated tissues these expression characteristics were lost. These observations would agree with the the above mechanism: studies in developing mice show that HMG-I/Y proteins are expressed at elevated levels in proliferating, relatively undifferentiated cells whereas somatic histone H1 is not detected until the four-cell stage. This higher ratio of HMG-I/Y to H1 in undifferentiated cells might be one parameter supporting the augmenting function of S/MARs. After differentiation occurred, augmentation could be restored for ear fibroblasts taken from the adult animal provided that they were kept proliferating prior to the assay. 3 Regional Demethylation The regulation of eukaryotic gene expression is a complicated process which involves the interaction of a large number of transacting factors with specific cis-regulatory elements. DNA methylation plays a role in this scheme by modulating protein-DNA interactions. Available evidence indicates that methylation serves to fix a transcriptionally silent state, after the assembly of repressive nucleoprotein complexes. Since the discovery of a demethylase, demethylation is seen as an active enzymatic process, controlled by specific cis regulatory elements (Bhattachrya, 1999). In the immune system DNA methylation plays multiple roles, most prominently in expression and gene rearrangement, which are both controlled by enhancer-S/MAR combinations. Transgenic models have shown that the µ- and κ chain S/MARs complement the intronic enhancers in the demethylation reaction, presumably through some form of synergy. While the µ enhancer alone can establish local areas of accessible chromatin, the S/MARs extend accessibility to more distal positions. These properties held true even when the specific interactions between enhancer- and 7 promoter-bound factors were interrupted by linking µ enhancer-S/MAR fragments to sites for bacteriophage RNA polymerases which were either close to or distal from the enhancer. The long-range accessibility was shown to correlate with extended demethylation of the gene construct but not necessarily with its active transcription. (Jenuwein et al., 1997) Similarly, the intronic enhancer together with its associated 3´S/MAR is necessary for demethylating the κ locus during B-cell differentiation. Replacing the κ-S/MAR by element `IA or by a plant S/MAR demonstrated that any S/MAR sequence in that position can take over this role, while tissue specificity is mediated by NF-κB sequences within the intronic enhancer. While enhancers and S/MARs appear to operate as inducers of chromatin accessibility and stimulators of transcription, demethylation seems not to be a secondary result of local promoter activity as it occurs also in the absence of nearby promoter sequences. These results suggest that the enhancer-S/MAR complex has two related but basically separate functions: to induce regional demethylation and to permit RNA synthesis (Kirillov et al., 1996). Several lines of evidence suggest that DNA demethylation also participates in recombination which is a prelude to proper expression. It is likely that the same combination of elements first allows access to the recombination machinery - perhaps concomitant with germline Jκ transcription. Following rearrangement, the same sequences are used a second time for demethylation and activating the newly juxtaposed Vκ-promoter. Is there any evidence that the natural loci behave differently from transgenes? Shulman and coworkers have described a gene-targeting approach by which the endogenous IgH locus can be modified in hybridoma cells. For the resulting cell lines expression of the IgH locus was found to depend strongly on the S/MAR-Eµ-S/MAR segment. Using this system, Wiersma et al. (1999; see also references therein) described the initially unexpected observation that expression of the endogenous µ-gene persists at half the normal level if either the enhancer or the S/MARs are retained. These results suggest that the roles of Eµ and S/MAR in initiating expression might be different from their role in maintaining expression. While both elements might be required to effect demethylation during initiation, either one may suffice to protect from a re-methylation event. Which are the domain-opening events that are triggered by regional demethylation? The possibility that histone H1 binds preferentially to DNA containing 5-methylcytosine in the dinucleotide CpG and would cause domain opening after its release by demethylation (IVA2) is appealing but is without experimental support (Campoy et al., 1995). An alternative model is based on methylation-specific interactions with methyl CpG binding proteins (MeCPs) that are used to repress certain genes. Two of these proteins, MeCP2 and MDBP-2 contain putative DNA binding motifs similar to those found in H1 and/or HMG-I/Y and thereby MeCPs may be specialized histone H1-like proteins. Interestingly, cloning an `attachment region binding proteinA (ARBP) revealed that this prototype S/MAR binder is homologous to MeCP2 and thereby seems to be a 8 multifunctional factor with roles in looped domain organization, the structure of pericentromeric heterochromatin and DNA methylation (Weitzel et al., 1997 and references therein). Occurrence of a protein that can recognize a single CpG but also associates with a wide range of S/MARs (especially if these contain a GC-rich core flanked by AT rich sequences) might suggest a reciprocal binding to various types of DNA. An opening mechanism could therefore involve capturing of ARBP by adjacent S/MARs due to rearrangements within a previously quiescent domain. This would expose CpGs to the action of demethylases, which would in turn remove an epigenetic mark typical for the inactive state. B Transcriptional Level 1 S/MARs as Topological Sinks Fig. 2 has demonstrated that S/MARs are composed from multiple destabilized sites. We will describe below the ways by which a BUR can be recognized by a variety of proteins, among these components with single-strand recognition capacity. S/MARs unwind as a consequence of stress which might be imposed by supercoiling or by the association of a protein factor. The observation that S/MARs can be reacted with single-strand specific agents in the living cell (review: Bode et al., 1998) suggests that they have acquired status `CA in Fig. 4. This state is induced by the association of single-strand-binders causing transient superhelical tension (B) which after a while is resolved by topoisomerases. In state C the S/MAR functions as a repository of underwound DNA which can be utilized in a dual fashion by the transcriptional machinery. If the element is localized in the positively supercoiled part of the twin domain and the superhelical strain is strong enough, part or all interactions with the ssDNA binding protein components are broken, the superhelicity is immediately relieved and ongoing transcription as well as re-initiation are facilitated (E). If the S/MARs occur downstream, but not far enough, from the transcription initiation site, the positive superhelicity is inadequate to break these contacts and transcription is stalled as for constructs Sp-a and Sp-b in Fig. 3. The same restriction does not appear to exist for a promoter-proximal S/MAR in the negatively supercoiled part of the twin-domain (cf. construct Sp5´3´). Here initiation may generally profit from the underwound structure stored in the S/MAR. During an induction of the human interferon-ß gene we could observe disappearance of CAAreactivity from the S/MAR region and simultaneous gain of reactivity in the transcription unit (Bode et al., 1995). In line with such a `topological sinkA function of S/MARs their stimulatory effect can be blocked if they are separated by GC rich fragments from the promoter (Poljak et al., 1994). 2 S/MARs as Repositories for Specific Transcription/Replication Factors Boulikas (1995) has studied regulatory regions within several gene domains and found that S/MARs are frequently a mosaic of transcription factor binding sites with a local overrepresentation of some sites. Especially ATTA and ATTTA motifs are abundant in major classes of origins of replication (ORIs) and S/MARs and this is typical for elements controlled by homeodomain 9 proteins. In line with the fact that a fraction of homeodomain proteins is part of the nuclear matrix it has been suggested that this fraction is involved in the switching of genes during development by changing the attachment points of chromatin loops. A role of the nuclear matrix in gene switching during development is also supported by changes its the general protein composition (review: Stein et al., 1999). It is remarkable that there appears to exist a class of factors which interferes with productive S/MAR-scaffold associations. This is exemplified by NFµNR, a factor that silences the immunoglobulin heavy chain (IgH) enhancer in non-B cells (Zong and Scheuermann, 1995) and SATB1 which is a transcriptional suppressor (Kohwi-Shigematsu et al., 1997) with an apparent function in T-lymphocytes. 3 SAF-B, a Platform to Establish Transcription and Processing Complexes Scaffold attachment factors (SAFs) are those nuclear proteins that interact with S/MARs and can hence be detected by Southwestern blotting methods. A protein, designated SAF-B, is an abundant component of chromatin, and is expressed in all tissues. SAF-B was found to interact with the C-terminal domain of RNA polymerase II and with subset of serine/arginine-rich RNA processing factors (SR-proteins). Since SAF-B overexpression changes alternative splicing patterns and the activity of S/MAR- flanked reporter genes in vivo it has been proposed that the factor serves as a platform to assemble a 'transcriptosome complex' in the vicinity of actively transcribed genes and also participates in signal transduction pathways which influence splice-site selection (Nayler et al. 1998). A S/MAR-mediated transcriptosome assembly is an intriguing feature that may explain certain aspects of the augmentation phenomenon. However, SAF-B itself is not a component of the nuclear matrix itself and anchoring to this substructure has to occur by other components of the transcriptosome complex such as RNA Pol II or members of the hnRNP protein family. Whether this productive association implies major rearrangements of some constitutive S/MAR matrix contacts is an open question. 5 An Alternative: S/MARs as Targeting Elements in Gene Transfer Experiments The activity of cis-acting sequences is traditionally investigated by the concept of `reverse geneticsA. To this end constructs with or without the element in question are transfected in parallel experiments and the level of transcription is monitored, for S/MAR-experiments necessarily under stable expression conditions. Is this a reliable procedure for the study of anchoring elements? S/MARs are not only recombinogenic sequences, which under certain circumstances yield multiple copy integration for the associated construct (Bode et al., 1996) but they might also direct the transgene to the nuclear matrix and cause integration in to regions of the genome with preexisting scaffold-association potential. In this case the outcome of the result would also be determined by the integration site in addition to the intrinsic differences of the constructs that are compared. A 10 final answer to this question will only come after a careful characterization of many integration sites for various constructs and by investigating both classes of constructs at predefined genomic integration sites. Rather than using homologous recombination for the alteration of genes in their native environments (Wiersma et al., 1999) we are in the process of using site-specific recombinases (Cre, Flp) for the introduction of transgenes into predetermined sites or for the stepwise decomposition of complete gene domains at a given genomic location. Initial reports on this concept have been published (Bode et al., 1996, 1998; Iber et al., 1999). While these experiments are in progress, we have completed the investigation of S/MARs as parts of retroviral constructs (Schübeler et al., 1996, 1998 and Fig. 3). Besides integrating a single intact copy into the genome of the host cell, retroviruses have an intrinsic apparatus by which sites of an increased transcriptional potential are sensed and utilized (Mielke et al., 1996). We have therefore proposed retroviral vectors as an efficient means to introduce transgenes at sites with a transcriptional potential anticipating that the integration machinery will overcome any targeting potential of a cloned sequence. The available data clearly support the idea that at least part of the augmenting action of S/MARs is due to a cis-effect. 6 Episomes and S/MARs Viruses depend on host cell functions for coming alive. It is obvious that cellular functions that require a structural organization will also be used by the virus for transcription and replication. Replication of the small DNA tumor virus SV40 is an excellent example, because in addition to virion proteins, it codes for only a few regulatory proteins, the most important one being the SV40 large tumor (`large TA) antigen. Deppert and Schirmbeck (1995) have summarized the evidence that all major viral processes during the life cycle from viral DNA replication to virion formation occur within the structural systems of the nucleus, in particular the chromatin and the nuclear matrix. Large T antigen itself becomes a member of the nuclear matrix where it binds to the origin of replication (ORI) and starts the assembly of an initiation complex in concert with cellular factors. It might also mediate the known matrix association of SV40 minichromosomes which grants their replication and maintenance as episomes. Interestingly, the SV40 genome contains a S/MAR which is part of the large T coding region (Pommier et al., 1990). Upon deletion of the S/MAR the episomal status is no longer maintained leading to the integration of the crippled construct. We have recently demonstrated that for SV40 derivatives episomal functions can be restored by replacing the large-T coding region in an SV40 vector by S/MAR element `IA (Piechacek et al., 1999). This S/MAR mediates the association of the episome with the nuclear matrix and the metaphase scaffold, helps to utilize the centromer function of host cell chromosomes as well as replication function of the host. Interestingly, bovine papilloma virus (BPV) and Epstein Barr virus (EBV) use related strategies, i.e. the E1/E2 or the EBNA-1 proteins, resp., for chromosome attachment and episome maintenance (review: Calos, 1998). 11 Although the participation of a S/MAR has not been directly demonstrated for BPV, its role as a maintenance element during the construction of episomal vectors has become evident: the potential of BPV-derived episomal vectors was increased dramatically when a hybrid plasmid (BPV-BV1) was constructed which could be shuttled between E. coli and mouse cells. For this function it had to contain a 69% subfragment of BPV and a minimum of 2.7 kb eukaryotic `stabilizing sequenceA which had been found, by trial and error, in the large ß-globin intron. Lateron we have demonstrated that this sequence coincides with a S/MAR (Klehr and Bode, 1988 and references therein). In Burkitt lymphoma cells, the extrachromosomal EBV virus genome is stably associated with the nuclear scaffold and this association is effected by a S/MAR which includes the origin of latent viral replication (oriP). Cellular DNA polymerases replicate the latent genomes during early S-phase and in synchrony with host cell DNA. Replication and partitioning between the daughter cells are mediated by interactions of the S/MAR-ORI region with a latent viral protein, EBNA-1, and the nuclear matrix. Other important signals for EBV replication and latent gene expression are also found in close proximity to the S/MAR (Jankelevich et al., 1992). Treatment with phorbolester, butyrate and TGF-ß induces the EBV lytic cycle with concomitant use of the lytic origin of replication (oriLyt). During this induction, ori P association was shown to decrease while ori Lyt becomes specifically associated with the nuclear scaffold. These findings suggest that the dynamic matrix association of the two EBV ORIs might regulate viral gene expression (Mattia et al., 1999). Besides their established function in replication there is one convincing demonstration that S/MARs contained in replicating episomes also effect transcriptional augmentation. To define the elements of the Ig-κ gene involved in deregulation of the c-myc gene after translocation, Hörtnagel et al. (1995) have assembled different parts of the Ig-κ locus in an EBV-derived episomal vector. These experiments clearly showed that the S/MAR is required for the maximum c-myc activation observed in Burkitt lymphoma cells. In order to differentiate between S/MAR and enhancer functions, both elements were also tested in transient transfection experiments where the enhancers provided a 30 fold activation while the S/MAR alone reduced transcription to the background level. This study suggests that episomally replicating constructs allow the study the role of S/MARs in transcription and should therefore be useful for their detailed analysis. A complication may arise from the fact that these derivatives of vector pHEBo will, in addition, depend on the ORI-associated S/MAR. In summary, for an augmenting action S/MARs will either need the buildup of superhelical tension which is granted after integration or in the framework of a covalently closed circular structure and/or they require the ultimate, ordered chromatin structure which can only be established by replication. V Ubiquitous Components of the Nuclear Scaffold 12 The backbone of eukaryotic nuclei can be isolated and characterized according to protocols which have been optimized for the removal of soluble components (Berezney and Coffey, 1974; Mirkovich et al, 1984; Fey et al., 1986). The resulting skeleton resembles the nucleus in size and shape and three of its substructures can be discerned by electron microscopy: i - the lamina-pore complex at the nuclear periphery, ii - an intranuclear network of filaments with associated granular material and iii - residual nucleolar structures. As expected for an operationally defined entity, various procedures lead to matrix/scaffold preparations that differ to some extent in ultrastructure (Belgrader et al., 1991) while the vast majority of protein constituents appears to be identical (Ivanchenko and Avramova, 1992). Even more important, the DNA elements (S/MARs) which are characterized by a specific re-association with these structures in the presence of a vast excess of bacterial competitor DNA are mostly independent of the origin of the nuclear skeleton. Our laboratory has adapted the LIS-(lithium 3,5-diiodosalicylate-) based procedure for nuclei isolated from cultured mammalian cells and plant tissues (Kay and Bode, 1995). The resulting scaffolds are used for capturing scaffold attached regions under stringent conditions. Relative binding affinities are derived by an `equal fractions approach@ and referenced to an internal standard. The scaffolds resisting LIS extraction contain components from the three mentioned nuclear compartments, i.e. the lamins A-C (lamina), nucleolin (nucleolus) and IF-type proteins from the fibrogranular internal network. These IF-like proteins may even comprise another lamin fraction since intranuclear lamins are uncovered by extracting the chromatin (Hozak, 1995). If overexpressed, another IF-type S/MAR binder, NuMA, fills the nucleus with an ordered lattice which is stable to detergent extraction suggesting a major structural role for this protein in the architecture of the interphase nucleus (Gueth-Hallonet et al., 1998). A scaffold of this composition binds supercoiled plasmids and ssDNA independent of sequence. In addition, it binds linear dsDNA provided it contains a S/MAR element. Since all S/MAR assays are performed with linearized fragments, and characterized by competition with ssDNA, only components mediating this binding mode will be considered in the following. In doing so, we learned that the two predominant protein constituents of the scaffold, SAF-A and the lamins (including other IF-type proteins), fully account for the binding properties of a complete scaffold. Other S/MAR binding constituents like histone H1, nucleolin and possibly actin (Ivanchenko and Avramova, 1992) may contribute to the binding but they cause no major imprint on the specificity toward dsDNA. ARBP, another abundant protein, is a special case due to its particular solubility features. It is part of a nuclear matrix isolated by 2M NaCl but absent from LIS-scaffolds (Ludérus et al., 1992 and references therein). A SAF-Proteins: The SAF-Box 13 Mattern et al. (1997 and references therein) have identified the 21 most abundant proteins that are exclusively present in the internal nuclear network. In line with earlier reports (Nakayasu and Berezney 1991) many of these belong to the group of heterogenous nuclear ribonucleoproteins (hnRNPs) supporting a model in which the major protein constituents are involved in RNA metabolism, -packaging and -transport. A major member of this group, hnRNP-U has first been characterized as the most prominent attachment factor in LIS-extracted scaffolds (called SAF-A by Fackelmayer and Richter, 1994 and SP120 by Tsutsui et al., 1993). SAF-A associates with multiple S/MARs and UV-crosslinking experiments show that this established RNA binder is likewise associated with DNA in vivo. S/MAR binding is optimal, if AAT patches@ (short stretches of consecutive A´s and T´s), are distributed according to certain rules (Tsutsui, 1998). HeLa-cells contain about 2 million SAF-A proteins per nucleus, half of which associate with the nuclear matrix in a salt-resistant manner. The other half is either bound to hnRNP particles or resides in a DNAseI extractable fraction. In vitro, SAF-A shows a pronounced propensity to selfpolymerize and this state is required to recognize S/MAR DNA. The primary structure of SAF-A reflects its dual function as there are two independent nucleic acid binding domains, i - a Cterminal RNA/ssDNA binding domain (RGG box), and ii - a S/MAR specific 45 amino acids Nterminal domain, called ASAF-box@, which is split and inactivated during apoptosis. The SAF-box resembles a homeobox lacking the DNA recognition helix and represents the first characterized protein domain specifically recognizing S/MARs (Fackelmayer 1999, submitted). It recurs in several eukaryotic proteins, from yeast to man, among these SAF-B (see IVB3). For a cloned SAF-box, a characteristic association with S/MARs can only be demonstrated in pull-down experiments and in case a critical protein density is reached on the surface of Sepharose-beads. These data indicate a cooperative binding mode which is also typical for scaffold-S/MAR interactions (Kay and Bode, 1994): each individual domain interacts only weakly with a DNA element, which may be an UE according to Fig. 2. Only the simultaneous binding of multiple SAF-boxes will then confer a strong and at the same time specific interaction (Zuckerkandl and Villet, 1988). This model would explain the well known phenomenon that there are hardly any naturally occuring S/MARs below a critical length of 300 bp. Another feature typical for S/MARscaffold interactions could also be demonstrated, i.e. DNA binding is sensitive to distamycin (which binds to the minor groove of oligo(dA) @oligo(dT) tracts), but not to chromomycin (specific for the minor grove in GC-rich regions). The failure of ssDNA to compete for the interaction of S/MARs with SAF-boxes shows that many but not all criteria of scaffold-S/MAR interactions can be explained by SAF-box proteins. B Lamins and other Intermediate Filament Proteins Intermediate filament (IF) proteins are built according to a common tripartite structure: a central α-helical rod which is flanked, on both sides, by nonhelical domains with ssDNA binding 14 potential. IF proteins self-assemble into long polymers by coiled-coil formation between the rods. The spatial distribution of S/MAR binding centers in the nuclear scaffold has been studied by Ludérus et al. (1994 and references therein). S/MAR sites appear to be distributed equally over the peripheral nuclear lamina and the internal fibrogranular network. In case of the lamina, lamins B (and later A) have been determined as the major binding partners. The specificity of a S/MARlamin interaction could be confirmed with paracrystal-like lamin polymers which were prepared by dialyzing a lamin solution into a buffer of low ionic strength. Two different types of interaction were discovered and these appear to be related to different features of S/MARs. One type involves the minor groove of the DNA double strand and the second single strand regions. Both modes of association are interdependent as S/MAR binding is almost completely inhibited by the presence of single-stranded competitors. These characteristics resemble other intermediate filament proteins which, in addition to their intrinsic ssDNA binding property, gain S/MAR binding potential in their filamentous state (Traub and Shoeman, 1994). It is hypothesized, that in this state two ssDNA binding domains act in concert to recognize individual strands in the BURs (Fig. 4). It has been claimed by the respective authors, that either lamin B or SAF-A mirror the S/MAR-binding behavior of a complete scaffold. However, while both proteins display a comparable recognition profile for native and artificial S/MAR elements and respond similarly to competition by distamycin versus chromomycin, they clearly differ regarding the competition of S/MARs by ssDNA. The 50% competition limit found for the complete scaffold (Kay and Bode, 1994) may therefore reflect the contribution of these two major contributors. VI How other S/MAR-Binding Proteins Deal with BUR-Associated Structures The prediction of S/MARs from primary sequence data has met with unexpected difficulties since the major scaffold proteins recognize a structural consensus rather than a primary sequence. This has been exemplified above by the prototype S/MAR binders, SAF-A and lamin A/B. A prominent S/MAR-specific feature is a regular assembly of UEs (Fig. 2). The resulting baseunpairing regions have been shown to attract many S/MAR binding proteins which can be isolated by `BUR-affinity chromatographyA (review: Kohwi-Shigematsu et al., 1997). So far this procedure led to the recovery of a `special-AT-rich sequence binding proteinA (SATB1), nucleolin, a cancerassociated protein p114 with relation to PARP and to a complex between PARP and DNA-PK (Galande and Kohwi-Shigematsu, 1999 and references therein). Interestingly, Ku autoantigen, the DNA-binding subunit of DNA-PK, is also a prominent ORI-binding factor. Related recognition properties also assigned to BRIGHT (Herrscher et al., 1995) and mutant forms p53 (p53mut; Will et al., 1998 and references therein). Among these examples, SATB1, nucleolin, PARP/DNA-PK and p53mut appear to be prototype binders recognizing BURs according to radically different mechanisms. For SATB1, chemical interference assays demonstrated binding along the minor groove of 15 double stranded S/MARs with very little contact to the bases. Association was ascribed to a S/MAR-domain and a homeodomain which together direct the protein to a specific CUE within the IgH-associated BUR. SATB1 does not bind to nor is it competed off by ssDNA suggesting that the CUE is recognized indirectly through an altered sugar phosphate backbone structure. Binding of SATB1 may therefore prevent unwinding and suppress promoter/enhancer functions (KohwiShigematsu et al., 1997). As in the other cases, the CUEs consist of a special AT-rich sequence context in which one strand is well- mixed A's. T's and C's, excluding G's. Within S/MARs these AATC sequences@ typically occur in clusters and they show a rather close correlation with base unpairing properties (Krawetz and Bode, unpublished). SATB1 associates with quite a number of different established S/MARs and its binding has been used as an alternative means for the detection of S/MAR elements . Nucleolin binds to RNA and single-stranded DNA. Within S/MARs it recognizes the region of highest base-unpairing potential. In contrast to the highly selective binding of SATB1 to double-stranded S/MARs, nucleolin also accepts single strands of S/MAR-DNA and among these the T-rich strand is preferred. Its affinity for S/MARs is severalfold higher than to RNA and human telomere DNA which are other established binding partners. Both PARP and DNA-PK are usually activated by DNA strand breaks, they can be recovered as a complex and are implicated in DNA-repair, -recombination, -replication, and transcription. While it has been described that PARP and the DNA-binding subunit of DNA-PK associate with free DNA ends, the highly specific association with a BURs in closed circular supercoiled DNA substrates could recently be demonstrated; this interaction is abolished after and may therefore be regulated by ADP-ribosylation (Galande and Kohwi-Shigematsu, 1999). p53mut specifically interacts with an AATATATTT core-unwinding region and it catalyzes DNA strand separation when this motif is located within a structurally labile sequence environment. Since there is no recognition of individual single strands, it is concluded that the active process of strand separation provides a basis for its high-affinity. HMG proteins are other prominent factors with a binding preference for S/MARs. Proteins with multiple HMG boxes (prototypes: HMG-1/2) recognize irregular DNA structures in a sequence-nonspecific manner. A preferred substrate is cruciform DNA which is bound with an affinity exceeding B-DNA. Cruciforms are formed by intrastrand pairing at inverted repeats which are rather prominent S/MAR features (review: Bode et al., 1998). Since base unpairing is a prerequisite for cruciform formation, it directly depends on SIDD properties. An 11-residue motif named AAT-hook@, first detected in the HMG-I(Y) protein, represents a DNA-binding unit different from the known α-helix, ß-sheet and Zn++-finger motifs. AT hooks recognize the narrow minor groove of AT-rich double strands in a way resembling distamycin and netropsin. High-affinity binding sites contain several A/T-tracts separated by 6-8 base pairs. Once 16 again, motifs of this architecture are common in BURs. In summary, the majority of proteins associating with S/MARs are attracted by specific features of base unpairing regions such as particular dsDNA structures [SATB1, HMG I(Y), SAFA], the recognition of single strands individually (lamins, nucleolin) or conjointly (p53mut) or secondary structures depending on prior unpairing (HMG-1/2). Sometimes special structures and single strands are recognized by different domains on the same protein (SAF-A). Together with sequence-specific factors these proteins form a regulatory network in which the transcriptional state of a chromatin domain can be precisely adjusted. Acknowledgements We are most grateful to our colleagues Frank Fackelmayer (Konstanz), Steven Krawetz (Detroit) and Stefan Stamm (Martinsried) for intense discussions on the occasion of the Gene Therapy Molecular Biology meeting in Crete (August 1998) and for obtaining relevant data prior to publication. The excellent cooperation with Hans-Joachim Lipps and Armin Baiker (Universität Witten-Herdecke) on the principles of episomal replication is gratefully acknowledged. Development of the present concepts would not have been possible without the continuous input and encouragement over many years by Terumi Kohwi-Shigematsu (Lawrence-Berkeley Laboratory). Work from the authors labs was supported by an EU grant to JBO (BIO4-CT98-0203), DFG grants (Bo 419/5; Bo 419/6), Danish Cancer Society Grant 97-100-32 to CMI and a grant from the NIH to CJB. REFERENCES Auten J., Agarwal M., Chen JY, Sutton R, Plavec I (1999) and references therein: Effect of scaffold attachment region on transgene expression in retrovirus vector-transduced primary T cells and macrophages. Hum Gene Ther 10: 1389-1399. Belgrader P, Siegel A J, Berezney R (1991): A comprehensive study on the isolation and characterization of the HeLa S3 nuclear matrix. J Cell Sci 98: 281-291. Benham C, Kohwi-Shigematsu T, Bode J (1997): Stress- induced duplex DNA destabilization in scaffold/matrix attachment regions. J Mol Biol 274: 181-196. Berezney R, Mortillaro MJ, Ma H, Wei X. Samarabandu J. (1995) The nuclear matrix: A structural milieu for genomic function. Int Rev Cytol 162A: 1-65. Berezney R, Coffey DS (1974): Identification of a nuclear protein matrix. Biochem Biophys Res Comm 60: 1410-1417. Bhattacharya SK, Ramchandani S, Cervoni N, Szyf M (1999): A mammalian protein with specific demethylase activity for mCpG DNA. Nature (London) 397: 579-583. Bode J, KohwiY, Dickinson L, Joh RT, Klehr D, Mielke C, Kohwi-Shigematsu T (1992): Biological significance of unwinding capability of nuclear matrix- associating DNAs. Science 255: 195-197. Bode J, Schlake T, Ríos-Ramírez M, Mielke C, Stengert M, Kay V, Klehr-Wirth D (1995):Scaffold/MatrixAttached Regions (S/MARs): Structural Properties Creating Transcriptionally Active Loci. Int Rev Cytol 162A: 389-453. Bode J, Stengert-Iber M, Schlake T, Kay V, Dietz- Pfeilstetter A (1996): Scaffold/matrix-attached regions: Topological switches with multiple regulatory functions. Crit Rev Eukaryot Gene Expr 6: 115-138. Bode J, Bartsch J, Boulikas T, Iber M, Mielke C, Schübeler D, Seibler J, Benham C (1998): Transcription- 17 promoting genomic sites in mammalia: their elucidation and architectural principles. Gene Therapy and Mol Biol 1: 551580. Boulikas T (1995): Chromatin domains and prediction of MAR sequences. Int Rev Cytol 162A: 279-388. Calos MP (1998): Stability without a centromere. Proc Natl Acad Sci USA 95: 4084-4085. Campoy FJ, Meehan RR, McKay S, Nixon J, Bird A (1995): Binding of histone H1 to DNA is indifferent to methylation at CpG sequences. J Biol Chem 270: 26473- 26481. Deppert W, Schirmbeck, R (1995): The nuclear matrix and virus function. Int Rev Cytol 162A: 485-537. Fackelmayer FO, Richter A (1994): Purification of two isoforms of hnRNP-U and characterization of their nucleic acid binding activity. Biochemistry 33: 10416-10422. Fey EG, Krochkalnic G, Penman S (1986): The nonchromatin substructures of the nucleus: the ribonucleoprotein (RNP)-containing and RNP-depleted matrices analyzed by sequential fractionation and resinless section electron microscopy. J Cell Biol 102: 1654-1665. Galande S, Kohwi-Shigematsu T (1999): Poly(ADP-ribose) polymerase and Ku autoantigen form a complex and synergistically bind to matrix attachment sequences. J Biol Chem 274: 20521-20528. Gasser S M, Laemmli UK (1987): A glimpse at chromosomal order. Trends in Genetics 3: 16-22. Gueth-Hallonet C, Wang J, Harborth J, Weber K, Osborn M (1998). Induction of a regular nuclear lattice by overexpression of NuMA. Exp Cell Res 243: 434-452. Herrscher RF, Kaplan MH, Lelsz DL, Das C, Scheuermann R, Tucker PW. (1995): The immunoglobulin heavy-chain matrix-associating regions are bound by Bright: A B cell-specific trans-activator that describes a new DNA- binding protein family. Genes Dev 9 3067-3082. Hörtnagel K, Mautner J, Strobl LJ, Wolf DA, Christoph B, Geltinger, C Polack A (1995): The role of immunoglobulin kappa elements in c-myc activation. Oncogene 10:1393-1401. Hozak P, Sasseville AM -J, Raymond Y, Cook PR (1995): Lamin proteins form an internal nucleoskeleton as well as a peripheral lamina in human cells. J Cell Sci 108: 635- 644. Iber M, Schübeler D, Seibler J, Höxter M, Bode J (1999): Efficient FACS selection procedure for cells undergoing Flp-mediated site-specific conversions. Trends Genet (tto01668: 1-4). Ivanchenko M, Avramova Z (1992): Interaction of MAR- sequences with nuclear matrix proteins. J Cell Biochem 50: 190-200. Jankelevich S, Kolman JL, Bodnar J W, Miller G (1992): A nuclear matrix attachment region organizes the Epstein-Barr viral plasmid in Raji cells into a single DNA domain. EMBO J 11: 1165-1176. Jenuwein T, Forrester WC, Fernandez-Herrero LA, Laible G, Dull M, Grosschedl R (1997): Extension of chromatin accessibility by nuclear matrix attachment regions. Nature (London) 385: 269-272. Käs E, Poljak L, Adachi Y, Laemmli UK (1993): A model for chromatin opening: Stimulation of topoisomerase II and restriction enzyme cleavage of chromatin by distamycin. EMBO J. 12: 115-126. Kay V, Bode J (1994): Binding specificity of a nuclear scaffold: Supercoiled, single-stranded, and scaffoldattached- region DNA. Biochemistry, 33: 367-374. Kay V, Bode J (1995): Detection of scaffold-attached regions (SARs) by in vitro techniques; activities of these elements in vivo. Meth Mol Cell Biol 5:186-194. Kirillov A, Kistler B, Mostoslavsky R, Cedar H, Wirth T, Bergman Y (1996): A role for nuclear NF-KB in B- cell specific demethylation of the Ig kappa locus. Nature Genet 13:, 435-441. Klehr D, Bode J (1988): Comparative evaluation of bovine papilloma virus (BPV) vectors for the study of gene expression in mammalian cells. Mol Gen (Life Sci Adv ) 7: 47-52 Kohwi-Shigematsu T, Maass K, Bode J (1997): A thymocyte factor SATB1 suppresses transcription of stably integrated matrix-attachment region-linked reporter genes. Biochemistry 36: 12005-12010. 18 Kohwi-Shigematsu T, deBelle I, Dickinson LA, Galande S. KohwiY (1998): Identification of base-unpairing region-binding proteins and characterization of their in vivo binding sequences. Meth Cell Biol 53: 323-354. Krawetz SA, Kramer JA, McCarrey JR (1999): Reprogramming the male gamete genome: a window to successful gene therapy. Gene 234: 1-9. Ludérus MEE, Den Blaauwen, JL, De Smit OJB, Compton DA, Van Driel R (1994): Binding of matrix attachment regions to lamin polymers involves single-stranded regions and the minor groove. Mol Cell Biol 14, 62976305. Mattern KA, van Driel R, de Jong L (1997): Composition and structure of the internal nuclear matrix.. Nuclear Structure and Gene Expression 87-110. Michalowski SM, Allen GC, Hall GE, Thompson WF, Spiker S (1999): Characterization of randomly-obtained matrix attachment regions (MARs) from higher plants. Biochemistry in press. Mielke C, Kohwi Y, Kohwi-Shigematsu T, Bode J (1990): Hierarchical binding of DNA fragments derived from scaffold-attached regions: Correlations of properties in vitro and function in vivo. Biochemistry, 29, 7475-7485. Mielke C, Maass K, Tümmler M, Bode, J (1996): Anatomy of highly expressing chromosomal sites targeted by retroviral vectors. Biochemistry 35: 2239-2252. Mirkovitch J, Mirault ME, Laemmli UK (1984): Organization of the higher-order chromatin loop: specific DNA attachment sites on nuclear scaffold. Cell 39: 223-232. Nakayasu H, Berezney R (1991): Nuclear matrins: Identification of the major nuclear matrix proteins. Proc Natl Acad Sci USA 88: 10312-10316. Nayler O, Strätling W, Bourquin JP, Stagljar I, Lindemann L, Jasper H, Hartmann AM, Fackelmayer FO, Ullrich A, Stamm S (1998): SAF-B protein couples transcription and pre-mRNA splicing to SAR/MAR elements.. Nucl Acid Res,26: 3542-3549. Piechaczek C, Fetzer C, Baiker A, Bode J, Lipps HJ (1999): A vector based on the SV40 origin of replication and chromosomal S/MARs replicates episomally in CHO cells. Nucl Acid Res 27: 426-428. Poljak L, Seum C, Mattioni T, Laemmli UK (1994): SARs stimulate but do not confer position independent gene expression. Nucl Acid Res 22: 4386-4394. Pommier Y, Cockerill PN Kohn KW Garrard WT (1990). Identification within the simian virus 40 genome of a chromosomal loop attachment site that contains topoisomerase II cleavage sites. J Virol 64: 419-423. Schübeler D, Mielke C, Maass K, Bode J (1996). Scaffold/matrix-attached regions act upon transcription in a context-dependent manner. Biochemistry 35: 11160-11169. Schübeler D, Maass K, Bode J (1998): Retargeting of retroviral integration sites for the predictable expression of transgenes and the analysis of cis-acting sequences. Biochemistry 37: 11907-11914. Stein GS, VanWijnen AJ, Stein JL,Lian JB, Pockwinse SH, McNeil S (1999): Implications for interrelationships between nuclear architecture and control of gene expression under microgravity conditions. Faseb J 13: S157-S166. Strissel PL, Dann HA, Pomykala HM, Diaz MO, Rowley JD, Olopade OI (1998): Scaffold-associated regions in the human type I interferon gene cluster on the short arm of chromosome 9th Genomics 47: 217-229. Thompson EM, Christians E, Stinnakre M-G, Renard J-P (1994):Scaffold attachment regions stimulate HSP70.1 expression in mouse preimplantation embryos but not in differentiated tissues. Mol Cell Biol 14: 4694-4703. Traub P, Shoeman RL (1994): Intermediate filament proteins: ytoskeletal elements with gene-regulatory function?. Int Rev Cytol 154: 1-104. Tsutsui K (1998): Synthetic concatemers as artificial MAR: importance of a particular configuration of short ATtracts for protein recognition. Gene Ther Mol Biol 1: 581-590. Tsutsui K, Tsutsui K, Okada S, Watarai S, Seki S, Yasuda T, Shohmori T (1993): Identification and characterization of a nuclear scaffold protein that binds the matrix attachment region DNA. J Biol Chem, 268: 12886- 19 12894. Weitzel JM, Buhrmester H, Strätling W H (1997): Chicken MAR-binding protein ARBP is homologous to rat methyl- CpG-binding protein MeCP2nd. Mol Cell Biol 17: 5656-5666. Wiersma EJ, Ronai D, Berru M, Tsui FWL Shulman MJ (1999): Role of the intronic elements in the endogenous immunoglobulin heavy chain locus - Either the matrix attachment regions or the core enhancer is sufficient to maintain expression. J Biol Chem 274: 4858-4862. Will K, Warnecke G, Wiesmüller L, Deppert, W (1998): Specific interaction of mutant p53 with regions of MARs with a high potential for base unpairing. Proc Natl Acad Sci USA 95: 13681-13686 Zong RT, Scheuermann RH (1995): Mutually exclusive interaction of a novel matrix attachment region binding protein and the NF-µR enhancer repressor - Implications for regulation of immunoglobulin heavy chain expression. J Biol Chem 270: 24010-24018. Zuckerkandl E, Villet R (1988): Generation of high specificity of effect through low-specificity binding of proteins to DNA. FEBS Lett. 231, 291-298. 20 Figure 1: DNA Recognition Profile of a Nuclear Scaffold A nuclear scaffold has been prepared by LIS-(lithium 3,5-diiodosalicylate-) extraction of cultured murine cells. The reassociation of a pUC-based vector with an 800 bp S/MAR insert (`IVA) is studied in the presence of a vast (100 000x) excess of bacterial competitor DNA. Due to nucleolytic activities of a scaffold an initially supercoiled S/MAR vector (see total input, T) becomes first nicked, then linearized during the reassociation process. Traces P (pellet) and S (supernatant mark fragments which associate with the scaffold or remain unbound. The supercoil strongly associates with the scaffold (i.e. occurs in the pellet fraction as does a S/MAR-free control). Unlike the S/MAR-free control the S/MAR vector exhibits evident but minor affinity for the scaffold, i.e. it is distributed over the P- (15%) and S- (85%) fractions. Complete scaffold association after linearization is exclusively found for the S/MAR containing vector. Figure 2: Anatomy of a Prototype S/MAR: Stress-Induced Duplex Destabilization (SIDD) Although S/MARs are not necessarily AT-rich, they contain many AT-patches which form the basis of their local strand separation under stress. While stress in vivo can arise by various mechanisms, a convenient way of its standardization is the application of superhelical tension in the context of a bacterial plasmid (i.e. at superhelical densities of σ = -0.055). Under these conditions very restricted regions of the plasmids open and can be trapped by single-strand specific agents (see the peaks flanking the Ampicillin-resistance gene and the phage f1-ORI). Under the same conditions S/MARs exhibit a multitude of more or less regularly spaced unwinding elements (UEs). Among these the most prominent one (the one reacting preferentially with KMnO4; insert shows KMnO4 reactivity in the living cell) is called the `core-unwinding elementA (CUE). Altogether the UEs in a S/MAR form a `base-unpairing regionA (BUR). Figure 3: Transcriptional Augmentation: Relevance of Distance S/MAR-elements have been inserted at various positions along a bicistronic retroviral vector construct. If this construct is transfected and its expression monitored during the transient expression phase S/MARs are seen to exhibit a slightly negative effect throughout. If an authentic single copy of the construct is integrated by retroviral infection and its expression is studied, the initiation rate is significantly augmented relative to the control (Sp) if the S/MAR is at a certain distance downstream from the promoter (construct c) or downstream and upstream from the promoter (´double-copy´ vector 5´3´). At a position immediately downstream from the transcriptional initiation site S/MARs inhibit the passage of RNA polymerase. A possible mechanism is discussed in Fig. 4. Figure 4: Augmentation explained by SIDD-properties of S/MARs 21 At least some S/MARs are particularly accessible to single-strand specific agents like chloroacetaldehyde (CAA, Bode et al., 1997), OsO4 (Paul and Ferl, 1993) or single-strand specific nucleases (Targa et al. 1994, Iarovaia et al., 1995). The association of ss-specific proteins (triangles) would lead to strand separation within S/MARs and raise transient topological problems (B). Their ultimate resolution by topoisomerases leads to the scaffold-associated ground state (C). Transcription-dependent buildup of positive superhelicity can then be resolved by breaking some (or all) ssDNA contacts enabling the immediate initiation by other polymerases. Table 1: Ubiquitous S/MAR-Binding Proteins with a Relaxed Sequence Specificity (Prototype S/MAR-Binders) Prominent S/MAR binders have been listed and their binding specificity indicated. The binding of supercoils by the nuclear scaffold has been described before (Tsutsui et al., 1988; Kay and Bode, 1994) but is not the subject of the present study which describes the recognition of linearized S/MARs. It is evident that the specificity of the S/MAR-scaffold interaction is reflected by the major, not tissue-specific, protein constituents. 22 Protein Recognition Function Name Alternative Function Abundance per Nucleus (Scaffold) TopoII (Sc1) Enzymatic plus structural functions; Sc1xSc2 (=UB2) 3E5 (60-80% in metaphase) HMG I(Y) Nucleosome phasing Chromatin bound RNA scDN A ++ ssDNA - ds S/MAR + minor groove; bending protein bending protein 1E5 Chromatin bound ++ + + Cruciforms H1 25E6 (1-15%) ++ - + (S/TPXX) MeCP2 1E5 - + KD = 2E-10M Lamins A,B also: internal network 100% NuMA mitotic spindle maintenance 2E5 (most) Nucleolin rDNA transcript., rRNA packaging, ribosome assembly (variable) PARP DNA-repair; complex with DNAPK (Ku-antigen) 1E6 (50%) SAF-A hRNP-U; packaging of hnRNAs 2E6 (50%) SAF-B Platform for transcriptosome/ splicing complex assembly 1E5 (5%) Chromatin bound TOTAL SCAFFOLD + + + minor groove + 3 AT-hooks HMG 1,2 ARBP Else + end domains (S/TPXX) minor and major groove (GGTGT); methyl. CpGs; ds S/MAR>350 bp minor groove; 80-90% competition by ssDNA; noncooperative binding + + + BUR, T-rich strand KD = 9E-9M + + + (RGGbox) ++ ++ + BUR under superhel. tension + (SAF-Box) KD(min) = 3E-9M AT-patches in dsS/MARs>300 bp, no competition by ss DNA; cooperative binding + (SAF-Box) Interaction with spliceosomes and Pol II ++ BUR; 50% competition by ssDNA; dsS/MAR > 300 bp cooperative binding 23 24 25 26 27