* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download mTOR complex 2 (mTORC2) controls hydrophobic motif
Survey
Document related concepts
Secreted frizzled-related protein 1 wikipedia , lookup
G protein–coupled receptor wikipedia , lookup
Proteolysis wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
Western blot wikipedia , lookup
Ultrasensitivity wikipedia , lookup
Signal transduction wikipedia , lookup
Lipid signaling wikipedia , lookup
Two-hybrid screening wikipedia , lookup
Biochemical cascade wikipedia , lookup
Monoclonal antibody wikipedia , lookup
Paracrine signalling wikipedia , lookup
Mitogen-activated protein kinase wikipedia , lookup
Transcript
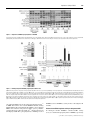
Biochem. J. (2008) 416, 375–385 (Printed in Great Britain) 375 doi:10.1042/BJ20081668 mTOR complex 2 (mTORC2) controls hydrophobic motif phosphorylation and activation of serum- and glucocorticoid-induced protein kinase 1 (SGK1) Juan M. GARCÍA-MARTÍNEZ1 and Dario R. ALESSI MRC Protein Phosphorylation Unit, College of Life Sciences, University of Dundee, Dow Street, Dundee DD1 5EH, Scotland, U.K. SGK1 (serum- and glucocorticoid-induced protein kinase 1) is a member of the AGC (protein kinase A/protein kinase G/protein kinase C) family of protein kinases and is activated by agonists including growth factors. SGK1 regulates diverse effects of extracellular agonists by phosphorylating regulatory proteins that control cellular processes such as ion transport and growth. Like other AGC family kinases, activation of SGK1 is triggered by phosphorylation of a threonine residue within the T-loop of the kinase domain and a serine residue lying within the C-terminal hydrophobic motif (Ser422 in SGK1). PDK1 (phosphoinositidedependent kinase 1) phosphorylates the T-loop of SGK1. The identity of the hydrophobic motif kinase is unclear. Recent work has established that mTORC1 [mTOR (mammalian target of rapamycin) complex 1] phosphorylates the hydrophobic motif of S6K (S6 kinase), whereas mTORC2 (mTOR complex 2) phosphorylates the hydrophobic motif of Akt (also known as protein kinase B). In the present study we demonstrate that SGK1 hydrophobic motif phosphorylation and activity is ablated in knockout fibroblasts possessing mTORC1 activity, but lacking the mTORC2 subunits rictor (rapamycin-insensitive companion of mTOR), Sin1 (stress-activated-protein-kinase-interacting protein 1) or mLST8 (mammalian lethal with SEC13 protein 8). Furthermore, phosphorylation of NDRG1 (N-myc downstream regulated gene 1), a physiological substrate of SGK1, was also abolished in rictor-, Sin1- or mLST8-deficient fibroblasts. mTORC2 immunoprecipitated from wild-type, but not from mLST8- or rictor-knockout cells, phosphorylated SGK1 at Ser422 . Consistent with mTORC1 not regulating SGK1, immunoprecipitated mTORC1 failed to phosphorylate SGK1 at Ser422 , under conditions which it phosphorylated the hydrophobic motif of S6K. Moreover, rapamycin treatment of HEK (human embryonic kidney)-293, MCF-7 or HeLa cells suppressed phosphorylation of S6K, without affecting SGK1 phosphorylation or activation. The findings of the present study indicate that mTORC2, but not mTORC1, plays a vital role in controlling the hydrophobic motif phosphorylation and activity of SGK1. Our findings may explain why in previous studies phosphorylation of substrates, such as FOXO (forkhead box O), that could be regulated by SGK, are reduced in mTORC2-deficient cells. The results of the present study indicate that NDRG1 phosphorylation represents an excellent biomarker for mTORC2 activity. INTRODUCTION Insulin and growth factors stimulate activation of SGK1 as well as other AGC kinases, such as Akt, S6K (S6 kinase), RSK (ribosomal S6K) and PKC (protein kinase C) isoforms by enhancing the phosphorylation of these enzymes at their T-loop kinase domain residue (Thr256 in SGK1), as well as at a C-terminal non-catalytic residue, termed the hydrophobic motif (Ser422 in SGK1). Previous studies have established that the activation of SGK1 and S6K is dependent on the activation of PI3K (phosphoinositide 3-kinase) and the production of the second messenger PtdIns(3,4,5)P3 [6,7]. This induces phosphorylation of SGK1 and S6K at its hydrophobic motif, promoting the interaction with PDK1 (phosphoinositidedependent kinase 1) [8,9]. PDK1 next activates SGK1 and S6K by phosphorylating the T-loop residues of these enzymes [8,9]. Consistent with this model, SGK1 and S6K activity is suppressed by inhibiting PI3K or by preventing PDK1 from interacting with the phosphorylated hydrophobic motif of SGK1 or S6K [10,11]. Activation and phosphorylation of Akt depends The SGK (serum- and glucocorticoid-induced protein kinase) isoforms are members of the AGC (protein kinase A/protein kinase G/protein kinase C) family kinases and their activity is stimulated by growth factors and other agonists [1,2]. There are three isoforms of SGK (SGK1, SGK2 and SGK3) that are widely expressed and are reported to possess distinct, as well as overlapping, roles to other AGC kinase family members such as Akt (also known as protein kinase B) [1,2]. One of the best characterized processes controlled by SGK involves its ability to stimulate sodium transport into epithelial cells by enhancing the stability and expression of the ENaC (epithelial sodium channel) (reviewed in [3]). This is achieved by SGK phosphorylating the NEDD4-2 (neural-precursor-cell-expressed developmentally down-regulated 4-2) ubiquitin E3 ligase, promoting its interaction with 14-3-3 proteins, thereby preventing it from binding to ENaC and targeting it for degradation [4,5]. Key words: Akt, mammalian target of rapamycin (mTOR) pathway, phosphoinositide 3-kinase (PI3K), serum- and glucocorticoid-induced protein kinase 1 (SGK1), S6 kinase. Abbreviations used: AGC, protein kinase A/protein kinase G/protein kinase C; ENaC, epithelial sodium channel; FOXO, forkhead box O; GSK3, glycogen synthase kinase 3; GST, glutathione transferase; HEK, human embryonic kidney; HRP, horseradish peroxidase; MEF, mouse embryonic fibroblast; mLST8, mammalian lethal with SEC13 protein 8; mTOR, mammalian target of rapamycin; mTORC, mTOR complex; NDRG1, N-myc downstream regulated gene 1; PDK1, phosphoinositide-dependent kinase 1; PH, pleckstrin homology; PI3K, phosphoinositide 3-kinase; PKC, protein kinase C; raptor, regulatory associated protein of mTOR; rictor, rapamycin-insensitive companion of mTOR; Protor, protein observed with rictor; RSK, ribosomal S6 kinase; SGK, serum- and glucocorticoid-induced protein kinase; Sin1, stress-activated-protein-kinase-interacting protein 1; S6K, S6 kinase; TBS, Tris-buffered saline; TSC2, tuberous sclerosis complex 2; WNK1, with no lysine (K) 1. 1 To whom correspondence should be addressed (email [email protected]). c The Authors Journal compilation c 2008 Biochemical Society 376 J. M. Garcı́a-Martı́nez and D. R. Alessi on PtdIns(3,4,5)P3 interacting with a PH (pleckstrin homology) domain on Akt that is not found on SGK1 or S6K. This induces a conformational change in Akt that enables PDK1 to phosphorylate the T-loop Thr308 residue [12–15]. PDK1 also contains a PH domain that binds with high affinity to PtdIns(3,4,5)P3 and other phosphoinositides which co-localizes PDK1 and Akt at the plasma membrane [15,16]. Binding of PDK1 to PtdIns(3,4,5)P3 is important for the activation of Akt, but not SGK1, as a knockin mutation that prevented PDK1 binding to PtdIns(3,4,5)P3 , suppressed the activation of Akt, but not SGK [17]. Recent work has established that complexes of the mTOR (mammalian target of rapamycin) protein kinase, termed mTORC1 (mTOR complex 1) and mTORC2 (mTOR complex 2), play a vital role in mediating the hydrophobic motif phosphorylation of Akt and S6K, as well as certain isoforms of PKC [18,19]. mTORC1 phosphorylates the hydrophobic motif residue of S6K (Thr389 ), whereas mTORC2 phosphorylates the hydrophobic motif of Akt (Ser473 ). mTORC1 consists of mTOR, raptor (regulatory associated protein of mTOR) and mLST8 (mammalian lethal with SEC13 protein 8; previously known as GβL). mTORC1 is activated by growth factors via a PI3Kregulated pathway, involving Akt-mediated phosphorylation of TSC2 (tuberous sclerosis complex 2) protein and PRAS40 (proline-rich Akt substrate of 40 kDa), which leads to the activation of the Rheb GTPase (reviewed in [20]). The activity of mTORC1 is also stimulated by nutrients such as amino acids via a distinct pathway involving the Rag GTPases binding to raptor [21,22]. mTORC1, and hence activation and phosphorylation of S6K, is also acutely inhibited by the macrolide rapamycin [23– 25]. mTORC2 consists of mTOR, rictor (rapamycin-insensitive companion of mTOR; also known as mAVO3), Sin1 (stressactivated-protein-kinase-interacting protein 1; also known as mSin1 or MIP1), mLST8 [26–31] and protor (protein observed with rictor) [32–34]. Unlike mTORC1, mTORC2 is insensitive to acute rapamycin treatment, although prolonged incubation disrupts mTORC2 assembly in certain cells [35]. mTORC2 is also activated by PI3K through an unknown mechanism, but, unlike mTORC1, its activity is not regulated by amino acids [26,27,29,36]. In the present study we explore whether mTOR complexes may play a role in regulating SGK1. Our results indicate that the hydrophobic motif phosphorylation, and hence activity of SGK1, is regulated by mTORC2. MATERIALS AND METHODS Materials Protein G–Sepharose, glutathione–Sepharose and [α-32 P]ATP were purchased from Amersham Biosciences. Pre-cast SDS polyacrylamide Bis-Tris gels and LipofectamineTM 2000 were from Invitrogen. Tween 20 and dimethyl pimelimidate were from Sigma, and CHAPS and rapamycin were from Calbiochem. PI103 was synthesized by Dr Natalia Shpiro at the University of Dundee. The wild-type control and mLST8-knockout MEFs (mouse embryonic fibroblasts) have been described previously [31] and were provided by Dr David Sabatini (Whitehead Institute for Biomedical Research, Cambridge, MA, U.S.A.). The wildtype control and Sin1-knockout MEFs have been described previously [30] and were provided by Dr Bing Su (Yale University School of Medicine, New Haven, CT, U.S.A.). The wild-type control and rictor-knockout MEFs have been described previously [37] and were provided by Dr Mark Magnuson (Vanderbilt University School of Medicine, Nashville, TN, U.S.A.). The muscle extracts from wild-type and SGK1-knockout mice have been described previously [17,38] and were provided by c The Authors Journal compilation c 2008 Biochemical Society Dr Krishna M. Boini and Dr Florian Lang (Department of Physiology, University of Tübingen, Tübingen, Germany). Antibodies The following antibodies were raised in sheep and affinitypurified on the appropriate antigen: anti-mLST8 (S837B, 3rd bleed) was raised against the human full-length mLST8 protein expressed in Escherichia coli (used for immunoblotting); antimTOR [S683B, 2nd bleed; residues 2–20 of human mTOR LGTGPAAATTAATTSSNVS, used for immunoblotting in HEK (human embryonic kidney)-293 cells and immunoprecipitation]; anti-protor-1 (S020C, 3rd bleed) was raised against the human full-length protor-1 protein expressed in E. coli (used for immunoblotting); anti-raptor (S682B, 3rd bleed; residues 1–20 of human raptor MESEMLQSPLLGLGEEDEAD, used for immunoblotting and immunoprecipitation); anti-rictor (S654B, 3rd bleed; residues 6–20 of human rictor RGRSLKNLRVRGRND, used for immunoblotting in HEK-293 cells and immunoprecipitation); anti-rictor (S274C, 1st bleed; residues 6– 20 of mouse rictor RGRSLKNLRIRGRND, used for immunoblotting); anti-Sin1 (S8C, 1st bleed) was raised against the human full-length Sin1 protein expressed in E. coli (used for immunoblotting); and anti-SGK1 phosphorylated at Thr256 (S987, 1st bleed; residues 251–262 of human SGK1 NSTTSTpFCGTPE, used for immunoblotting). An anti-NDRG1 (N-myc downstream regulated gene 1) antibody (S276B, 2nd bleed) was made in sheep using recombinant GST (glutathione transferase)fusion of full-length NDRG1 (used for immunoblotting). An antibody that recognizes NDRG1 phosphorylated at Thr346 , Thr356 and Thr366 (S911B, 2nd bleed; termed pNDRG1 3xThrP) was raised against the nonapeptide RSRSHpTSEG, whose sequence is common to all three sites (used for immunoblotting). Anti-Akt1 (S695B, 3rd bleed; residues 466–480 of human Akt1 RPHFPQFSYSASGTA, used for immunoblotting); antiS6K (S417B, 2nd bleed; residues 25–44 of human S6K1 AGVFDIDLDQPEDAGSEDEL, used for immunoblotting); and anti-S6K2 (S469A, 3rd bleed; residues 476–495 of human S6K2, RPPSGTKKSKRGRGRPGR, used for immunoblotting) were also used. Anti-GST (S902A, 1st bleed) antibody was raised against the GST tag expressed from pGex4T (used for immunoblotting). An anti-mTOR antibody used for immunoblotting of mouse mTOR in MEFs was purchased from Santa Cruz Biotechnology (catalogue number sc-1549). For phospho-immunoblotting of the hydrophobic motif of SGK1(Ser422 ), we employed the Thr389 S6K antibody (catalogue number 9205) from Cell Signaling Technology which we previously demonstrated crossreacted with the phosphorylated Ser422 of SGK1 [39]. For the immunoblotting of endogenous SGK1 in Figure 6, we employed the phospho-SGK1 Ser422 antibody from Santa Cruz Biotechnology (catalogue number sc-16745-R) and the antiSGK1 antibody from Upstate (catalogue number 07-315). The phospho-Akt Ser473 (catalogue number 9271), phospho-S6K1 Thr389 (catalogue number 9234), phospho-RSK Ser235 (catalogue number 4856) and total RSK (catalogue number 2217) antibodies, used for immunoblotting, were also purchased from Cell Signaling Technology. Secondary antibodies coupled to HRP (horseradish peroxidase) used for immunoblotting were obtained from Thermo Scientific. General methods Tissue culture, immunoblotting, restriction enzyme digests, DNA ligations and other recombinant DNA procedures were performed using standard protocols. DNA constructs used for transfection Regulation of SGK by mTORC2 377 were purified from E. coli DH5α using the Qiagen plasmid Mega or Maxi kit according to the manufacturer’s protocol. All DNA constructs were verified by DNA sequencing, which was performed by the Sequencing Service, School of Life Sciences, University of Dundee, Dundee, Scotland, U.K., using DYEnamic ET terminator chemistry (Amersham Biosciences) on Applied Biosystems automated DNA sequencers. MEFs were cultured with additional non-essential amino acids and 1 % sodium pyruvate solution. G–Sepharose using dimethyl pimelimidate. Immunoprecipitations were carried out for 1 h at 4 ◦C on a vibrating platform. The immunoprecipitates were washed four times with Hepes lysis buffer, followed by two washes with Hepes kinase buffer. The immunoprecipitates were resuspended in 30 μl of sample buffer (not containing 2-mercaptoethanol), filtered through a 0.22 μm Spin-X filter, and 2-mercaptoethanol to a concentration of 1 % (v/v) was added. Samples were subjected to electrophoresis and immunoblot analysis as described below. Buffers GST-pulldown of transfected SGK1 for immunoblot analysis The following buffers were used: Tris lysis buffer [50 mM Tris/ HCl (pH 7.5), 1 mM EGTA, 1 mM EDTA, 0.3 % CHAPS, 1 mM sodium orthovanadate, 10 mM sodium-β-glycerophosphate, 50 mM sodium fluoride, 5 mM sodium pyrophosphate, 0.27 M sucrose, 0.15 M NaCl, 0.1 % 2-mercaptoethanol, 1 mM benzamidine and 0.1 mM PMSF]; buffer A [50 mM Tris/HCl (pH 7.5), 0.1 mM EGTA and 0.1 % 2-mercaptoethanol]; Hepes lysis buffer [40 mM Hepes (pH 7.5), 120 mM NaCl, 1 mM EDTA, 0.3 % CHAPS, 10 mM sodium pyrophosphate, 10 mM sodium-βglycerophosphate, 50 mM sodium fluoride, 0.5 mM sodium orthovanadate, 1 mM benzamidine and 0.1 mM PMSF]; Hepes kinase buffer [25 mM Hepes (pH 7.5) and 50 mM KCl]; TBS (Tris-buffered saline)-Tween buffer [50 mM Tris/HCl (pH 7.5), 0.15 M NaCl and 0.1 % Tween 20]; and sample buffer [50 mM Tris/HCl (pH 6.8), 6.5 % (v/v) glycerol, 1 % (w/v) SDS and 1 % (v/v) 2-mercaptoethanol]. At 36 h post-transfection, HEK-293 cells, HeLa cells, MCF-7 cells or MEFs were lysed in Tris lysis buffer. Lysate (0.5–1 mg) was affinity-purified on glutathione–Sepharose. Incubations were carried out for 1 h at 4 ◦C on a vibrating platform. The resultant precipitates were then washed twice with Tris lysis buffer containing 0.5 M NaCl, followed by two washes with buffer A. The immunoprecipitates were resuspended in 20 μl of sample buffer, filtered through a 0.22 μm Spin-X filter, and samples were subjected to electrophoresis and immunoblot analysis as described below. Amounts of GST–SGK1 were assessed as described below using LI-COR scanning methodology. Cell lysis HEK-293 cells, HeLa cells, MCF-7 cells or MEFs were cultured and treated as described in the legends to the Figures. Following treatment, cells were rinsed twice with ice-cold PBS and then lysed using Tris lysis buffer. Whole-cell lysates were centrifuged (18 000 g at 4 ◦C for 20 min), supernatants were removed and stored in aliquots at − 80 ◦C until required. Plasmids and transfection A full-length cDNA encoding human SGK1 from an infant brain library was obtained from the IMAGE (Integrated Molecular Analysis of Genomes and their Expression) Consortium (clone ID42669). DNA constructs encoding full-length SGK1 or the truncated form of SGK1 (NSGK1) lacking the N-terminal 60 residues, as well as the point mutations in the SGK1 gene, have been described previously [6]. For transfection studies, typically ten 10-cm-diameter dishes of HEK-293 or HeLa cells were cultured and each dish was transfected with 5–10 μg of the indicated plasmids using the polyethylenimine method [40]. MCF-7 cells were transfected with 5 μg of the indicated plasmids using LipofectamineTM 2000 as described by the manufacturer. MEFs were transfected with 10 μg of the indicated plasmids using the MEF Nucleofector® Kits 1 and 2 as described by the manufacturer (Amaxa). Electroporation was performed using the A-23 nucleofector program (Amaxa). Immunoprecipitation of endogenous mTOR complexes for immunoblot analysis HEK-293 cells or MEFs were lysed in Hepes lysis buffer. Lysate (1– 4 mg) was pre-cleared by incubating with 5–20 μl of Protein G–Sepharose conjugated to pre-immune IgG. The lysate extracts were then incubated with 5–20 μl of Protein G–Sepharose conjugated to 5–20 μg of the indicated antibodies or preimmune IgG. All antibodies were covalently conjugated to Protein mTOR complexes kinase assays HEK-293 cells or MEFs were freshly lysed in Hepes lysis buffer. Lysate (1–4 mg) was pre-cleared by incubating with 5– 20 μl of Protein G–Sepharose conjugated to pre-immune IgG. The lysate extracts were then incubated with 5–20 μl of Protein G–Sepharose conjugated to 5–20 μg of either mTOR, rictor, raptor or pre-immune IgG. All antibodies were covalently conjugated to Protein G–Sepharose. Immunoprecipitations were carried out for 1 h at 4 ◦C on a vibrating platform. The immunoprecipitates were washed four times with Hepes lysis buffer, followed by two washes with Hepes kinase buffer. For raptor immunoprecipitates used for phosphorylating S6K1 and SGK1 (Figures 4E and 4F), the buffer for the initial two wash steps included 0.5 M NaCl to ensure that we obtained optimal kinase activity [41]. GST– NSGK1 (4 μg of total protein, of which 35 % is GST–SGK1) was isolated from serum-deprived HEK-293 cells incubated with PI-103 (1 μM for 1 h). GST–S6K1 (0.5 μg) was purified from serum-deprived HEK-293 cells incubated with rapamycin (0.1 μM for 1 h). mTOR reactions were initiated by adding 0.1 mM ATP and 10 mM Mg2+ in the presence or absence of GST–NSGK1 (4 μg of total protein, of which 35 % is GST– SGK1) or GST–S6K1 (0.5 μg of total protein). Reactions were carried out for 60 min at 30 ◦C on a vibrating platform and stopped by the addition of SDS sample buffer. Reactions were then filtered through a 0.22 μm Spin-X filter and samples were subjected to electrophoresis and immunoblot analysis. SGK1 kinase assay HEK-293 cells or MEFs were lysed in Tris lysis buffer. Lysate (50–200 μg) was incubated with 5–20 μl of glutathione– Sepharose for 1 h at 4 ◦C on a vibrating platform. SGK1 activity was assayed exactly as described previously [6] using the Crosstide peptide (GRPRTSSFAEG) at 30 μM. Incorporation of [32 P]-phosphate into the peptide substrate was determined by applying the reaction mixture on to P81 phosphocellulose paper and scintillation counting after washing the papers in phosphoric acid. One unit of activity was defined as that which catalysed the incorporation of 1 nmol of [32 P]-phosphate into the substrate. For the activity assay of purified SGK1 after phosphorylation with mTORC2 and/or PDK1 (Figure 3C), GST–NSGK1 was c The Authors Journal compilation c 2008 Biochemical Society 378 J. M. Garcı́a-Martı́nez and D. R. Alessi phosphorylated with mTORC2 (as described above) in the presence or absence of purified GST–PDK1 (0.1 μg), which was added during the last 10 min of the reaction. Independent aliquots (1 μg) of the resultant phosphorylated GST–NSGK1 were then assayed for SGK1 activity using the Crosstide assay described above. Quantifying the amount of GST–SGK1 The amount of GST–SGK1 isolated from MEF cells, as well as HEK-293 cells, was quantified by undertaking quantitative Coomassie Blue staining of SDS gels in which GST–SGK1 precipitates were run side-by-side with known amounts of BSA markers. The gels were analysed using a LI-COR Odyssey IR detection system following the manufacturer’s guidelines. The band intensity was quantified using LI-COR software. Immunoblotting Total cell lysate (20 μg) or immunoprecipitated samples were heated at 95 ◦C for 5 min in sample buffer, and subjected to PAGE and electrotransferred on to nitrocellulose membranes. Membranes were blocked for 1 h in TBS-Tween buffer containing 10 % (w/v) dried skimmed milk powder. The membranes were probed with the indicated antibodies in TBS-Tween containing 5 % (w/v) dried skimmed milk powder or 5 % (w/v) BSA for 16 h at 4 ◦C. Detection was performed using HRP-conjugated secondary antibodies and ECL (enhanced chemiluminescence) reagent. RESULTS Indication that mTORC2 regulates SGK1 hydrophobic motif phosphorylation To explore whether mTORC2 regulates SGK1, we first expressed a form of SGK1 lacking the N-terminal PEST degradation motif [6,42] in wild-type and rictor-deficient MEFs that lack mTORC2 activity [37]. Cells were cultured in the presence of serum and SGK1 activity, as well as hydrophobic motif Ser422 phosphorylation, was analysed. In wild-type cells, SGK1 was active (∼ 2 units/mg GST–SGK1) and significantly phosphorylated at its hydrophobic motif. Mutation of Ser422 to alanine abolished recognition of SGK1 by the phospho-Ser422 antibody as well as SGK1 activity. In contrast, in rictor-knockout MEFs, SGK1 was expressed at a similar level to wild-type cells, but was inactive and not detectably phosphorylated at its hydrophobic motif (Figure 1A). In agreement with the rictor-deficient cells lacking mTORC2 activity, Akt was not phosphorylated at its hydrophobic motif (Ser473 ). However, the rictor-knockout cells still possessed mTORC1 function, as S6K was still phosphorylated at its hydrophobic motif (Thr389 ) (Figure 1A). Activation of SGK1 is triggered by the interaction of PDK1 with SGK1 following the phosphorylation of Ser422 in the hydrophobic motif. An SGK1 mutant in which the hydrophobic motif Ser422 residue is mutated to aspartate, in order to mimic phosphorylation, is active when expressed in cells owing to its ability to constitutively interact with PDK1 [6,10,43]. It would therefore be expected that expression of the SGK1[S422D] mutant in rictor-knockout cells should bypass the requirement for TORC2 in activating this enzyme. Consistent with this, we found that SGK1[S422D], in contrast with wild-type SGK1, was significantly active when expressed in rictor-deficient cells (Figure 1B). Full-length SGK1, although expressed at lower levels than SGK1 lacking the PEST motif, was also phosphorylated at Ser422 in wild-type, but not in rictor-deficient, MEFs (Figure 1C). We c The Authors Journal compilation c 2008 Biochemical Society Figure 1 mTORC2-deficient MEFs lack SGK1 activity and hydrophobic motif phosphorylation (A and B) Rictor wild-type (wt) and Rictor-knockout (ko) cells were transfected with the indicated DNA constructs encoding GST–NSGK1. Cells were cultured in the presence of 10 % (v/v) foetal bovine serum to maintain PI3K pathway activity and were lysed 36 h post-transfection. SGK1 was affinity-purified on glutathione–Sepharose and subjected to immunoblot analysis with the indicated antibodies and also assayed for activity using the Crosstide peptide substrate. The amount of GST–SGK1 immunoprecipitated in each assay was quantified following electrophoresis on SDS/PAGE and Coomassie Blue staining as described in the Materials and methods section. Histograms are the mean specific activity + − S.E.M. from three different samples, with each sample assayed in duplicate. Cell lysates were also immunoblotted with the indicated antibodies for non-SGK blots. (C) As in (A), except that cells were transfected with constructs expressing full-length SGK1. (D and E) As in (A), except that mLST8 wild-type (wt) and mLST8-knockout (ko) (D) or Sin1 wild-type (wt) and Sin1-knockout (ko) (E) MEFs were used. Similar results were obtained in three independent experiments. U/mg, units/mg. Regulation of SGK by mTORC2 Figure 2 379 Dependence of NDRG1 phosphorylation on mTORC2 Immunoblot analysis was undertaken with the indicated antibodies from control wild-type (wt) or knockout (ko) MEFs cultured in the presence of 10 % (v/v) foetal bovine serum to maintain PI3K pathway activity. Cell extracts derived from the skeletal muscle of wild-type or SGK1-knockout mice were also analysed. Immunoblots are representative of three different experiments. Figure 3 Immunoprecipitated mTORC2 phosphorylates SGK1 in vitro (A) HEK-293 cell lysates were subjected to immunoprecipitation (IP) with an anti-rictor or pre-immune IgG antibody. Immunoprecipitates were immunoblotted with the indicated antibodies raised against different mTORC1 and/or mTORC2 components. (B) Anti-rictor or pre-immune IgG immunoprecipitates from HEK-293 cell lysates were incubated with dephosphorylated GST–NSGK1 in the presence of MgATP for 60 min and then subjected to immunoblot analysis with the antibodies indicated. (C) As in (B), except that samples were assayed in the presence (+) or absence (−) of PDK1. The catalytic activity of GST–SGK1 towards the Crosstide peptide was also measured. The amount of GST–SGK1 present in each assay was quantified following SDS/PAGE and Coomassie Blue staining as described in the Materials and methods section. Histograms represent the mean specific activity + − S.E.M. from three different samples, with each sample assayed in duplicate. (D) As in (A), except that cell lysates were derived from mLST8 control wild-type (wt) or mLST8-knockout (ko) MEFs. (E) As in (B), except that cell lysates were derived from mLST8 control wild-type (wt) or mLST8-knockout (ko) MEFs. Similar results were obtained in three independent experiments. also found that SGK1 was not detectably phosphorylated at Ser422 in MEFs that lack the other critical mTORC2 subunits, namely mLST8 (Figure 1D) or Sin1 (Figure 1E). Similar to rictor-deficient MEFs and consistent with previous studies [28–31], mLST8and Sin1-knockout cells lacked Akt Ser473 phosphorylation, but still displayed S6K Thr389 phosphorylation, confirming a lack of mTORC2, but not mTORC1, activity in these cells (Figures 1D and 1E). Evidence that mTORC2 regulates activity of endogenous SGK1 To investigate whether mTORC2 controlled SGK1 activity in vivo, we examined the phosphorylation of NDRG1, a previously c The Authors Journal compilation c 2008 Biochemical Society 380 Figure 4 J. M. Garcı́a-Martı́nez and D. R. Alessi Immunoprecipitated mTORC1 phosphorylates S6K1, but not SGK1, in vitro (A) mTOR was immunoprecipitated from rictor control wild-type (wt) and rictor-knockout (ko) cells that are deficient in mTORC2, but still possess mTORC1. Immunoprecipitates were immunoblotted with the antibodies indicated. (B and C) mTOR immunoprecipitates were incubated in the presence (+) or absence (−) of dephosphorylated GST–S6K1 (B) or GST–NSGK1 (C) in the presence of MgATP for 60 min and then subjected to immunoblot analysis with the antibodies indicated. (D–F) HEK-293 cell extracts were subjected to immunoprecipitation with pre-immune IgG, anti-raptor or anti-rictor antibodies. The immunoprecipitates were immunoblotted with the antibodies indicated (D). The immunoprecipitates were also incubated in the presence (+) or absence (−) of dephosphorylated GST–S6K1 (E) or GST–NSGK1 (F) in the presence of MgATP for 60 min and then subjected to immunoblot analysis with the antibodies indicated. IP, immunoprecipitation. characterized substrate for SGK1 [44]. SGK1 phosphorylates NDRG1 at three residues, Thr346 , Thr356 and Thr366 , that lie within a repeated decapeptide sequence [44]. To ensure that this approach represented a reliable readout for SGK1 activity, we first studied the phosphorylation of NDRG1 in skeletal muscle derived from wild-type and SGK1-knockout, mice and found that NDRG1 was phosphorylated in extracts derived from wild-type, but not SGK1-knockout, mice (Figure 2). We then examined NDRG1 phosphorylation in MEFs deficient in rictor, mLST8 or Sin1, and observed that NDRG1 was markedly phosphorylated in all of the wild-type control MEFs, but not in any of the mTORC2-subunitdeficient cells (Figure 2). mTORC2 phosphorylates the hydrophobic motif of SGK1 in vitro We next isolated endogenous mTORC2 complex from HEK-293 cells by immunoprecipitating rictor and investigated whether it was capable of phosphorylating the hydrophobic motif of SGK1. The rictor immunoprecipitates contained the known mTORC2 complex subunits (mTOR, rictor, mLST8, Sin-1 and protor-1), but not the specific mTORC1 component raptor (Figure 3A). In the presence of MgATP and recombinant SGK1 (isolated from serum-starved HEK-293 cells incubated with a PI3K inhibitor), immunoprecipitated mTORC2 induced phosphoryl c The Authors Journal compilation c 2008 Biochemical Society ation of Ser422 (Figure 3B). In parallel experiments, a control immunoprecipitation undertaken with a pre-immune antibody, failed to phosphorylate SGK1 at Ser422 (Figure 3B). mTORC2 did not phosphorylate SGK1 at its T-loop Thr256 residue, whereas PDK1 phosphorylated SGK1 at its T-loop, but not the hydrophobic motif (Figure 3C). Phosphorylation of SGK1 with mTORC2 in the absence of PDK1 did not stimulate SGK1 activity significantly (Figure 3C), consistent with the evidence that phosphorylation of Thr256 is required to trigger activation of SGK1 [6]. Incubation of SGK1 with PDK1 in the absence of mTORC2 induced substantial activation of SGK1 to an activity of ∼ 3 units/mg GST–SGK1 (Figure 3C), similar to the specific activity of wildtype SGK1 expressed in MEFs (Figure 1A). In the presence of both mTORC2 and PDK1, SGK1 was phosphorylated at both Thr256 and Ser422 and its activity was further stimulated to 5– 6 units/mg GST–SGK1 (Figure 3C). To demonstrate that an intact mTORC2 complex is required to catalyse hydrophobic motif phosphorylation of SGK1, we immunoprecipitated rictor from either wild-type or mLST8knockout MEFs and tested how this affected phosphorylation of SGK1 at Ser422 . Consistent with previous work [31], we found that the lack of mLST8 abolished the interaction of rictor with mTOR, without affecting the ability of rictor to interact with Sin1 and protor (Figure 3D). Rictor immunoprecipitates derived from Regulation of SGK by mTORC2 381 mLST8-deficient cells failed to phosphorylate SGK1 under conditions which rictor immunoprecipitated from wild-type control MEFs phosphorylated SGK1 at Ser422 (Figure 3E). Immunoprecipitated mTORC1 phosphorylates the hydrophobic motif of S6K, but not SGK1 To investigate whether the mTORC1 complex was capable of phosphorylating SGK1 at Ser422 , we immunoprecipitated mTOR from rictor-knockout MEFs that lack mTORC2, but still possess a functional mTORC1 complex, as emphasized by the observations that S6K is still phosphorylated in these cells (Figure 1). Consistent with previous studies [31,37], immunoprecipitates of mTOR derived from rictor-deficient MEFs were associated with the mTORC1 components raptor and mLST8, but not with the mTORC2 subunits rictor, Sin1 and protor-1 (Figure 4A). mTOR immunoprecipitated from rictor-deficient MEFs phosphorylated S6K at Thr389 in vitro to the same extent as mTOR isolated from wild-type cells, in agreement with mTORC1 mediating this reaction (Figure 4B). However, in parallel experiments, mTOR immunoprecipitated from rictor-knockout MEFs failed to phosphorylate SGK1 at Ser422 under conditions which mTOR immunoprecipitated from wild-type MEFs phosphorylated SGK1 (Figure 4C). We also isolated mTORC1 by immunoprecipitating raptor from HEK-293 cells and demonstrated that it was associated with mTOR and mLST8, but not with rictor, Sin-1 or protor-1 (Figure 4D). Consistent with the conclusion that mTORC1 is unable to phosphorylate SGK1 at Ser422 , raptor immunoprecipitates failed to phosphorylate SGK1 at Ser422 , under conditions which they phosphorylated S6K at Thr389 (Figures 4E and 4F). In parallel experiments, rictor immunoprecipitates phosphorylated SGK1 at Ser422 , but did not phosphorylate the hydrophobic motif of S6K (Figures 4E and 4F). Phosphorylation of the hydrophobic motif of SGK1 is not inhibited by the mTORC1 inhibitor rapamycin To study whether mTORC1 has any role in regulating hydrophobic motif phosphorylation of SGK1 in vivo, we investigated how the activation and phosphorylation of SGK1 was affected by shortterm treatment of HEK-293 cells with the mTORC1 inhibitor rapamycin, conditions that do not affect mTORC2 [27]. HEK293 cells expressing full-length SGK1 were cultured in serum and treated in the presence or absence of rapamycin (100 nM) for 30 min. As expected, rapamycin abolished mTORC1-regulated phosphorylation of S6K at Thr389 , as well as phosphorylation of the S6 protein substrate at Ser235 , without affecting mTORC2mediated phosphorylation of Akt at Ser473 (Figure 5). However, rapamycin did not significantly inhibit SGK1 activity, its phosphorylation at Ser422 or phosphorylation of its NDRG1 substrate (Figure 5). As expected, treatment of cells with the PI3K inhibitor PI-103, which also inhibits mTORC1 and mTORC2 [45], suppressed SGK1 activity and phosphorylation of Ser422 (Figure 5). Consistent with this, PI-103 also inhibited the phosphorylation of NDRG1, Akt, S6K and the S6 protein. A recent study by Hong et al. [46], reported that in MCF-7 and HeLa cells, hydrophobic motif phosphorylation of SGK1 is controlled by mTORC1 and thus is inhibited by rapamycin. To analyse this further, we expressed full-length SGK1 in MCF-7 cells (Figure 5B) as well as in HeLa cells (Figure 5C) and tested whether phosphorylation of the hydrophobic motif of SGK1 or NDRG1 was inhibited by rapamycin. In contrast with the findings of Hong et al. [46], we observed that phosphorylation of SGK1 at Ser422 or phosphorylation of NDRG1 was not Figure 5 The mTORC1 inhibitor rapamycin does not suppress hydrophobic motif phosphorylation or activation of SGK1 in three different cell lines HEK-293 (A), MCF-7 (B) and HeLa (C) cells were transfected with a DNA construct encoding GST–SGK1 (full-length enzyme). Cells were cultured in the presence of 10 % (v/v) foetal bovine serum in order to maintain PI3K pathway activity. At 36 h post-transfection, cells were left treated for 30 min in the presence (+) or absence (−) of 1 μM PI-103 or 100 nM rapamycin. Cells were lysed, SGK1 was affinity-purified on glutathione–Sepharose and either subjected to immunoblot analysis with the antibodies indicated (A–C) or its catalytic activity assessed employing the Crosstide substrate (A). The amount of GST–SGK1 immunoprecipitate present in each assay was quantified following SDS/PAGE and Coomassie Blue staining as described in the Materials and methods section. Histograms represent the mean specific activity + − S.E.M. from three different samples, with each sample assayed in duplicate. Cell lysates were also analysed by immunoblotting with the indicated non-SGK antibodies (A–C). Immunoblots are representative of three different experiments. affected by rapamycin under conditions which this drug inhibited hydrophobic motif phosphorylation of S6K at Thr389 . As expected, phosphorylation of SGK1 at Ser422 in MCF-7 and HeLa cells was abolished following treatment with PI-103. c The Authors Journal compilation c 2008 Biochemical Society 382 Figure 6 J. M. Garcı́a-Martı́nez and D. R. Alessi Analysis of endogenous phosphorylation of SGK Lysates from the cell lines indicated generated as described in the legend for Figure 5 were electrophoresed on SDS/PAGE (10 % gels) and then subjected to immunoblot analysis with the antibodies indicated. The Santa Cruz Biotechnology phospho-Ser422 -SGK1 antibody (catalogue number sc-16745-R) was the same as that used in the study by Hong et al. [46]. Positions of molecular mass markers from Bio-Rad (catalogue number 161-0373) are shown. AB, antibody. Hong et al. [46] utilized an anti-phospho-Ser422 -SGK1 antibody (catalogue number sc-16745-R; Santa Cruz Biotechnology) to analyse the phosphorylation of endogenous SGK1. We have purchased this antibody and immunoblotted MCF-7, HeLa and HEK293 cell extracts utilized in the studies shown in Figure 5. The Santa Cruz Biotechnology antibody recognizes a significant non-specific band that migrates at a similar position to GST– SGK1 (∼ 85 kDa), making it hard to assess hydrophobic motif phosphorylation of overexpressed GST–SGK1 (Figure 6). However, the Santa Cruz Biotechnology phospho-Ser422 -SGK1 antibody also strongly recognizes a phosphorylated protein of ∼ 70 kDa whose phosphorylation is inhibited by both rapamycin and PI-103 (Figure 6). In our opinion, this protein is likely to comprise endogenous S6K1 and/or S6K2, rather than SGK1, as this signal co-migrates with the band recognized by antibodies c The Authors Journal compilation c 2008 Biochemical Society against S6K1/S6K2 protein. Immunoblotting with an antibody that recognizes endogenous SGK1 demonstrates that it migrates at the expected ∼ 48 kDa position (Figure 6). Following a long exposure of the immunoblot, the Santa Cruz Biotechnology phospho-Ser422 -SGK1 antibody weakly recognizes bands of ∼ 45–55 kDa that are likely to represent endogenous SGK1 and/or other isoforms/splice variants of SGK (Figure 6). Importantly, phosphorylation of proteins attributed to endogenous SGK isoforms was inhibited by PI-103, but not by rapamycin (Figure 6). DISCUSSION The results of the present study demonstrate that mTORC2 phosphorylates the hydrophobic motif of SGK1. This is based on the finding that in MEFs lacking the critical mTORC2 subunits, Regulation of SGK by mTORC2 Figure 7 Mechanism of SGK1 activation In response to insulin and growth factors PI3K (PI-3 kinase), by an unknown mechanism, stimulates the phosphorylation of SGK1 at its hydrophobic motif via mTORC2. This phosphorylation does not directly activate SGK1, but enables PDK1 to interact with SGK1 through its PIF-pocket docking site, thereby inducing T-loop phosphorylation and activation of SGK1. SGK1 is not phosphorylated at its hydrophobic motif and is thus inactive (Figure 1). Moreover, NDRG1 is not phosphorylated at the residues targeted by SGK1 in mTORC2-deficient cells (Figure 2). MEFs lacking active mTORC2 still possessed functional mTORC1 activity as emphasized by S6K being phosphorylated in these cells. However, despite possessing mTORC1 activity, SGK1 was not detectably phosphorylated at its hydrophobic motif, suggesting that mTORC1 does not contribute to the phosphorylation of SGK1, at least in MEFs. This conclusion is also consistent with our present observations (Figures 5 and 6), as well as previous reports [6,7,47], that acute rapamycin treatment of HEK-293, HeLa or MCF-7 cells does not inhibit serum-induced SGK1 activity or phosphorylation, under conditions which it inhibited phosphorylation and activation of S6K. To further demonstrate that mTORC2 controls SGK1, we found that immunoprecipitated mTORC2 can phosphorylate SGK1 at Ser422 in vitro and that this phosphorylation is dependent upon the presence of the rictor and mLST8 subunits of mTORC2 (Figure 3). Although isolated endogenous mTORC1 phosphorylated the hydrophobic motif of S6K, in a parallel reaction it did not phosphorylate the hydrophobic motif of SGK1 (Figure 4). We propose a model in Figure 7 in which growth factors and other agonists that stimulate PI3K lead to the activation of mTORC2. Activated mTORC2 phosphorylates the hydrophobic motif of SGK1 triggering its interaction with PDK1, via the PIF-motif substrate-docking site. PDK1 then phosphorylates the T-loop of SGK1 resulting in its activation. This model accounts for the sensitivity of SGK1 activation to PI3K inhibitors [6,7], but not rapamycin. It is also explains why prior hydrophobic motif phosphorylation and integrity of the PIF-pocket of PDK1 is essential for SGK1 activation and phosphorylation [10,11]. It also accounts for why the binding of PtdIns(3,4,5)P3 to PDK1 is not required for the activation of SGK1 in vivo [17], as phospho- 383 inositide binding is not required for PDK1 to interact with the hydrophobic motif of SGK1 and phosphorylate its T-loop residue [43]. One study reported that the WNK1 [with no lysine (K) 1] kinase, which is activated by osmotic shock and regulates salt uptake into cells [48], activated SGK1 by controlling the hydrophobic phosphorylation of SGK1 [49]. How this fits into the model of SGK1 activation shown in Figure 7 is unclear, unless WNK1 plays a role in controlling the activity of mTORC2. Hong et al. [46] have recently reported, in disparity to the observations made in the present study, that the phosphorylation of the hydrophobic motif of SGK1 was regulated by mTORC1. Key evidence to support this conclusion was based on the finding that phosphorylation of the hydrophobic motif of SGK1 was sensitive to the mTORC1 inhibitor rapamycin in WM35, MCF-7 and HeLa cells [46]. However, we have been unable to reproduce these findings and observe that phosphorylation of the hydrophobic motif of SGK1 is not inhibited by treatment of HEK-293, MCF-7 or HeLa cells with rapamycin, under conditions where phosphorylation of the hydrophobic motif of S6K1 is inhibited (Figure 5). Other groups have also reported that SGK1 activation in HEK-293 or HeLa cells is not inhibited by rapamycin [6,7,47]. We have found that the Santa Cruz Biotechnology phospho-Ser422 SGK1 antibody used in the Hong et al. [46] study recognizes phosphorylated endogenous S6K1 and/or S6K2 (∼ 70 kDa) on immunoblot analysis of cell extracts much more strongly than it recognizes endogenous phosphorylated SGK1 (∼ 48 kDa) (Figure 6). Long exposure of immunoblots was required to detect hydrophobic motif phosphorylation of endogenous SGK in MCF7 or HeLa cells that was inhibited by PI-103, but not by rapamycin (Figure 6). To avoid confusion when employing phospho-Ser422 SGK1 antibodies, caution is required to discriminate between the lower abundance phosphorylation of SGK1 migrating at ∼ 48 kDa compared with the much stronger recognition of S6K1/S6K2 migrating at ∼ 70 kDa. There seems to be significant antigenic similarity between the phosphorylated hydrophobic motifs of SGK1 and S6K1, as antibodies raised against the phosphorylated hydrophobic motif of S6K1 also recognize the phosphorylated hydrophobic motif of SGK1 [39]. A key implication of the present findings is that mTORC2deficient cells investigated in the present study are devoid of SGK1 activity, and therefore the phosphorylation of cellular substrates of SGK1 should be markedly reduced. In contrast with SGK1, Akt is still activated to a significant extent in mTORC2deficient cells [30,31,37], as it is phosphorylated at Thr308 by PDK1 in a reaction that is not dependent upon mTORC2. In this regard, phosphorylation of well-characterized Akt substrates, such as GSK3 (glycogen synthase kinase 3) and TSC2, was not observed to be significantly impaired following the loss of critical mTORC2 subunits in Sin1- [30] or mLST8- [31] deficient MEFs. This is probably due to partial activation of Akt being capable of inducing near-normal phosphorylation of its substrates. In contrast with TSC2 and GSK3, the phosphorylation of FOXO (forkhead box O) 1 (Thr24 ) and FOXO3a (Thr32 ) was markedly reduced in cells lacking mTORC2 activity [30,31]. This observation was interpreted in one study to imply that Akt phosphorylated at only Thr308 may possess a different substrate specificity to Akt phosphorylated at both Thr308 and Ser473 [30]. However, an alternative explanation suggested by the present study, is that the lack of SGK activity in mTORC2-deficient cells accounts for inhibition of FOXO phosphorylation. Consistent with this, previous reports have demonstrated that SGK isoforms are capable of efficiently phosphorylating FOXO [50,51]. Moreover, employing an antibody that recognizes substrates phosphorylated at an Akt/SGK phosphorylation motif, Jacinto et al. [30] reported that an non-identified protein of 48 kDa was heavily phosphorylated c The Authors Journal compilation c 2008 Biochemical Society 384 J. M. Garcı́a-Martı́nez and D. R. Alessi in wild-type, but not Sin1-knockout, MEFs. It is tempting to speculate that the protein visualized in this experiment was in fact NDRG1, that possesses a molecular mass of ∼ 48 kDa. Undertaking a screen to identify proteins whose phosphorylation is markedly suppressed in mTORC2-deficient cells would be a good approach to identify SGK substrates. Identification of novel SGK substrates would expand our understanding of the roles of these poorly characterized kinases. There is also much on-going research to develop drugs that suppress the activity of mTORC2 for the treatment of cancer [19]. The present study suggests that specific mTORC2 inhibitors will be more effective in suppressing phosphorylation of SGK1 substrates than Akt substrates. In conclusion, we have identified SGK1 as a novel substrate for the mTORC2 complex. We demonstrate that mTORC2 phosphorylates SGK1 at Ser422 and that this is required for its SGK1 activation. Our results indicate that the SGK-phosphorylated form of NDRG1 represents an excellent biomarker of mTORC2 activity and that mTORC2-deficient cells could be utilized to identify substrates of SGK. ACKNOWLEDGEMENTS We thank Dr Krishna M. Boini and Dr Florian Lang (Department of Physiology, University of Tübingen, Tübingen, Germany), Dr Mark Magnuson (Vanderbilt University School of Medicine, Nashville, TN, U.S.A.), Dr David Sabatini (Whitehead Institute for Biomedical Research, Cambridge, MA, U.S.A.) and Dr Bing Su (Yale University School of Medicine, New Haven, CT, U.S.A.) for the provision of reagents. We are also grateful to the Sequencing Service (School of Life Sciences, University of Dundee, Dundee, Scotland, U.K.) for DNA sequencing, the Post Genomics and Molecular Interactions Centre for Mass Spectrometry facilities (School of Life Sciences, University of Dundee, Dundee, Scotland, U.K.) and the protein production and antibody purification teams [DSTT (Division of Signal Transduction Therapy), University of Dundee, Dundee, Scotland, U.K.] co-ordinated by Hilary McLauchlan and James Hastie for the expression and purification of antibodies. FUNDING This work was supported by the Medical Research Council and AstraZeneca (a grant to J. M. G. M.). REFERENCES 1 Lang, F., Bohmer, C., Palmada, M., Seebohm, G., Strutz-Seebohm, N. and Vallon, V. (2006) (Patho)physiological significance of the serum- and glucocorticoid-inducible kinase isoforms. Physiol. Rev. 86, 1151–1178 2 Tessier, M. and Woodgett, J. R. (2006) Serum and glucocorticoid-regulated protein kinases: variations on a theme. J. Cell. Biochem. 98, 1391–1407 3 Loffing, J., Flores, S. Y. and Staub, O. (2006) Sgk kinases and their role in epithelial transport. Annu. Rev. Physiol. 68, 461–490 4 Debonneville, C., Flores, S. Y., Kamynina, E., Plant, P. J., Tauxe, C., Thomas, M. A., Munster, C., Chraibi, A., Pratt, J. H., Horisberger, J. D. et al. (2001) Phosphorylation of Nedd4-2 by Sgk1 regulates epithelial Na+ channel cell surface expression. EMBO J. 20, 7052–7059 5 Ichimura, T., Yamamura, H., Sasamoto, K., Tominaga, Y., Taoka, M., Kakiuchi, K., Shinkawa, T., Takahashi, N., Shimada, S. and Isobe, T. (2005) 14-3-3 proteins modulate the expression of epithelial Na+ channels by phosphorylation-dependent interaction with Nedd4-2 ubiquitin ligase. J. Biol. Chem. 280, 13187–13194 6 Kobayashi, T. and Cohen, P. (1999) Activation of serum- and glucocorticoid-regulated protein kinase by agonists that activate phosphatidylinositide 3-kinase is mediated by 3phosphoinositide-dependent protein kinase-1 (PDK1) and PDK2. Biochem. J. 339, 319–328 7 Park, J., Leong, M. L., Buse, P., Maiyar, A. C., Firestone, G. L. and Hemmings, B. A. (1999) Serum and glucocorticoid-inducible kinase (SGK) is a target of the PI 3kinase-stimulated signaling pathway. EMBO J. 18, 3024–3033 8 Mora, A., Komander, D., Van Aalten, D. M. and Alessi, D. R. (2004) PDK1, the master regulator of AGC kinase signal transduction. Semin. Cell. Dev. Biol. 15, 161–170 9 Biondi, R. M. (2004) Phosphoinositide-dependent protein kinase 1, a sensor of protein conformation. Trends Biochem. Sci. 29, 136–142 c The Authors Journal compilation c 2008 Biochemical Society 10 Collins, B. J., Deak, M., Arthur, J. S., Armit, L. J. and Alessi, D. R. (2003) In vivo role of the PIF-binding docking site of PDK1 defined by knock-in mutation. EMBO J. 22, 4202–4211 11 Collins, B. J., Deak, M., Murray-Tait, V., Storey, K. G. and Alessi, D. R. (2005) In vivo role of the phosphate groove of PDK1 defined by knockin mutation. J. Cell Sci. 118, 5023–5034 12 Milburn, C. C., Deak, M., Kelly, S. M., Price, N. C., Alessi, D. R. and Van Aalten, D. M. (2003) Binding of phosphatidylinositol 3,4,5-trisphosphate to the pleckstrin homology domain of protein kinase B induces a conformational change. Biochem. J. 375, 531–538 13 Alessi, D. R., Deak, M., Casamayor, A., Caudwell, F. B., Morrice, N., Norman, D. G., Gaffney, P., Reese, C. B., MacDougall, C. N. and Harbison, D. et al. (1997) 3-Phosphoinositide-dependent protein kinase-1 (PDK1): structural and functional homology with the Drosophila DSTPK61 kinase. Curr. Biol. 7, 776–789 14 Stokoe, D., Stephens, L. R., Copeland, T., Gaffney, P. R., Reese, C. B., Painter, G. F., Holmes, A. B., McCormick, F. and Hawkins, P. T. (1997) Dual role of phosphatidylinositol-3,4,5-trisphosphate in the activation of protein kinase B. Science 277, 567–570 15 Calleja, V., Alcor, D., Laguerre, M., Park, J., Vojnovic, B., Hemmings, B. A., Downward, J., Parker, P. J. and Larijani, B. (2007) Intramolecular and intermolecular interactions of protein kinase B define its activation in vivo . PLoS Biol. 5, e95 16 Currie, R. A., Walker, K. S., Gray, A., Deak, M., Casamayor, A., Downes, C. P., Cohen, P., Alessi, D. R. and Lucocq, J. (1999) Role of phosphatidylinositol 3,4,5-trisphosphate in regulating the activity and localization of 3-phosphoinositide-dependent protein kinase-1. Biochem. J. 337, 575–583 17 Bayascas, J. R., Wullschleger, S., Sakamoto, K., Garcia-Martinez, J. M., Clacher, C., Komander, D., van Aalten, D. M., Boini, K. M., Lang, F., Lipina, C. et al. (2008) Mutation of the PDK1 PH domain inhibits protein kinase B/Akt, leading to small size and insulin resistance. Mol. Cell. Biol. 28, 3258–3272 18 Wullschleger, S., Loewith, R. and Hall, M. N. (2006) TOR signaling in growth and metabolism. Cell 124, 471–484 19 Guertin, D. A. and Sabatini, D. M. (2007) Defining the role of mTOR in cancer. Cancer Cell 12, 9–22 20 Li, Y., Corradetti, M. N., Inoki, K. and Guan, K. L. (2004) TSC2: filling the GAP in the mTOR signaling pathway. Trends Biochem. Sci. 29, 32–38 21 Sancak, Y., Peterson, T. R., Shaul, Y. D., Lindquist, R. A., Thoreen, C. C., Bar-Peled, L. and Sabatini, D. M. (2008) The Rag GTPases bind raptor and mediate amino acid signaling to mTORC1. Science 320, 1496–1501 22 Kim, E., Goraksha-Hicks, P., Li, L., Neufeld, T. P. and Guan, K. L. (2008) Regulation of TORC1 by Rag GTPases in nutrient response. Nat. Cell Biol. 10, 935–845 23 Loewith, R., Jacinto, E., Wullschleger, S., Lorberg, A., Crespo, J. L., Bonenfant, D., Oppliger, W., Jenoe, P. and Hall, M. N. (2002) Two TOR complexes, only one of which is rapamycin sensitive, have distinct roles in cell growth control. Mol. Cell 10, 457–468 24 Hara, K., Maruki, Y., Long, X., Yoshino, K., Oshiro, N., Hidayat, S., Tokunaga, C., Avruch, J. and Yonezawa, K. (2002) Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell 110, 177–189 25 Kim, D. H., Sarbassov, D. D., Ali, S. M., King, J. E., Latek, R. R., Erdjument-Bromage, H., Tempst, P. and Sabatini, D. M. (2002) mTOR interacts with raptor to form a nutrient-sensitive complex that signals to the cell growth machinery. Cell 110, 163–175 26 Jacinto, E., Loewith, R., Schmidt, A., Lin, S., Ruegg, M. A., Hall, A. and Hall, M. N. (2004) Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat. Cell Biol. 6, 1122–1128 27 Sarbassov, D. D., Ali, S. M., Kim, D. H., Guertin, D. A., Latek, R. R., Erdjument-Bromage, H., Tempst, P. and Sabatini, D. M. (2004) Rictor, a novel binding partner of mTOR, defines a rapamycin-insensitive and raptor-independent pathway that regulates the cytoskeleton. Curr. Biol. 14, 1296–1302 28 Yang, Q., Inoki, K., Ikenoue, T. and Guan, K. L. (2006) Identification of Sin1 as an essential TORC2 component required for complex formation and kinase activity. Genes Dev. 20, 2820–2832 29 Frias, M. A., Thoreen, C. C., Jaffe, J. D., Schroder, W., Sculley, T., Carr, S. A. and Sabatini, D. M. (2006) mSin1 is necessary for Akt/PKB phosphorylation, and its isoforms define three distinct mTORC2s. Curr. Biol. 16, 1865–1870 30 Jacinto, E., Facchinetti, V., Liu, D., Soto, N., Wei, S., Jung, S. Y., Huang, Q., Qin, J. and Su, B. (2006) SIN1/MIP1 maintains rictor–mTOR complex integrity and regulates Akt phosphorylation and substrate specificity. Cell 127, 125–137 31 Guertin, D. A., Stevens, D. M., Thoreen, C. C., Burds, A. A., Kalaany, N. Y., Moffat, J., Brown, M., Fitzgerald, K. J. and Sabatini, D. M. (2006) Ablation in mice of the mTORC components raptor, rictor, or mLST8 reveals that mTORC2 is required for signaling to Akt-FOXO and PKCα, but not S6K1. Dev. Cell 11, 859–871 Regulation of SGK by mTORC2 32 Pearce, L. R., Huang, X., Boudeau, J., Pawlowski, R., Wullschleger, S., Deak, M., Ibrahim, A. F., Gourlay, R., Magnuson, M. A. and Alessi, D. R. (2007) Identification of Protor as a novel Rictor-binding component of mTOR complex-2. Biochem. J. 405, 513–522 33 Thedieck, K., Polak, P., Kim, M. L., Molle, K. D., Cohen, A., Jeno, P., Arrieumerlou, C. and Hall, M. N. (2007) PRAS40 and PRR5-like protein are new mTOR interactors that regulate apoptosis. PLoS ONE 2, e1217 34 Woo, S. Y., Kim, D. H., Jun, C. B., Kim, Y. M., Haar, E. V., Lee, S. I., Hegg, J. W., Bandhakavi, S., Griffin, T. J. and Kim, D. H. (2007) PRR5, a novel component of mTOR complex 2, regulates platelet-derived growth factor receptor β expression and signaling. J. Biol. Chem. 282, 25604–25612 35 Sarbassov, D. D., Ali, S. M., Sengupta, S., Sheen, J. H., Hsu, P. P., Bagley, A. F., Markhard, A. L. and Sabatini, D. M. (2006) Prolonged rapamycin treatment inhibits mTORC2 assembly and Akt/PKB. Mol. Cell 22, 159–168 36 Sarbassov, D. D., Guertin, D. A., Ali, S. M. and Sabatini, D. M. (2005) Phosphorylation and regulation of Akt/PKB by the rictor–mTOR complex. Science 307, 1098–1101 37 Shiota, C., Woo, J. T., Lindner, J., Shelton, K. D. and Magnuson, M. A. (2006) Multiallelic disruption of the rictor gene in mice reveals that mTOR complex 2 is essential for fetal growth and viability. Dev. Cell 11, 583–589 38 Wulff, P., Vallon, V., Huang, D. Y., Volkl, H., Yu, F., Richter, K., Jansen, M., Schlunz, M., Klingel, K., Loffing, J. et al. (2002) Impaired renal Na+ retention in the sgk1-knockout mouse. J. Clin. Invest. 110, 1263–1268 39 Lizcano, J. M., Deak, M., Morrice, N., Kieloch, A., Hastie, C. J., Dong, L., Schutkowski, M., Reimer, U. and Alessi, D. R. (2002) Molecular basis for the substrate specificity of NIMA-related kinase-6 (NEK6). Evidence that NEK6 does not phosphorylate the hydrophobic motif of ribosomal S6 protein kinase and serum- and glucocorticoid-induced protein kinase in vivo . J. Biol. Chem. 277, 27839–27849 40 Durocher, Y., Perret, S. and Kamen, A. (2002) High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells. Nucleic Acids Res. 30, E9 41 Sancak, Y., Thoreen, C. C., Peterson, T. R., Lindquist, R. A., Kang, S. A., Spooner, E., Carr, S. A. and Sabatini, D. M. (2007) PRAS40 is an insulin-regulated inhibitor of the mTORC1 protein kinase. Mol. Cell 25, 903–915 385 42 Belova, L., Sharma, S., Brickley, D. R., Nicolarsen, J. R., Patterson, C. and Conzen, S. D. (2006) Ubiquitin-proteasome degradation of serum- and glucocorticoid-regulated kinase-1 (SGK-1) is mediated by the chaperone-dependent E3 ligase CHIP. Biochem. J. 400, 235–244 43 Biondi, R. M., Kieloch, A., Currie, R. A., Deak, M. and Alessi, D. R. (2001) The PIF-binding pocket in PDK1 is essential for activation of S6K and SGK, but not PKB. EMBO J. 20, 4380–4390 44 Murray, J. T., Campbell, D. G., Morrice, N., Auld, G. C., Shpiro, N., Marquez, R., Peggie, M., Bain, J., Bloomberg, G. B., Grahammer, F. et al. (2004) Exploitation of KESTREL to identify N-myc downstream-regulated gene family members as physiological substrates for SGK1 and GSK3. Biochem. J. 384, 477–488 45 Raynaud, F. I., Eccles, S., Clarke, P. A., Hayes, A., Nutley, B., Alix, S., Henley, A., Di-Stefano, F., Ahmad, Z., Guillard, S. et al. (2007) Pharmacologic characterization of a potent inhibitor of class I phosphatidylinositide 3-kinases. Cancer Res. 67, 5840–5850 46 Hong, F., Larrea, M. D., Doughty, C., Kwiatkowski, D. J., Squillace, R. and Slingerland, J. M. (2008) mTOR-raptor binds and activates SGK1 to regulate p27 phosphorylation. Mol. Cell 30, 701–711 47 Auld, G. C., Campbell, D. G., Morrice, N. and Cohen, P. (2005) Identification of calcium-regulated heat-stable protein of 24 kDa (CRHSP24) as a physiological substrate for PKB and RSK using KESTREL. Biochem. J. 389, 775–783 48 Richardson, C. and Alessi, D. R. (2008) The regulation of salt transport and blood pressure by the WNK-SPAK/OSR1 signalling pathway. J. Cell Sci. 121, 3293–3304 49 Xu, B. E., Stippec, S., Chu, P. Y., Lazrak, A., Li, X. J., Lee, B. H., English, J. M., Ortega, B., Huang, C. L. and Cobb, M. H. (2005) WNK1 activates SGK1 to regulate the epithelial sodium channel. Proc. Natl. Acad. Sci. U.S.A. 102, 10315–10320 50 Brunet, A., Park, J., Tran, H., Hu, L. S., Hemmings, B. A. and Greenberg, M. E. (2001) Protein kinase SGK mediates survival signals by phosphorylating the forkhead transcription factor FKHRL1 (FOXO3a). Mol. Cell. Biol. 21, 952–965 51 Tullet, J. M., Hertweck, M., An, J. H., Baker, J., Hwang, J. Y., Liu, S., Oliveira, R. P., Baumeister, R. and Blackwell, T. K. (2008) Direct inhibition of the longevity-promoting factor SKN-1 by insulin-like signaling in C. elegans . Cell 132, 1025–1038 Received 15 August 2008/6 October 2008; accepted 17 October 2008 Published as BJ Immediate Publication 17 October 2008, doi:10.1042/BJ20081668 c The Authors Journal compilation c 2008 Biochemical Society