* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download carboxylic acids esters amides (R
Asymmetric induction wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Hydroformylation wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Sulfuric acid wikipedia , lookup
Hofmann–Löffler reaction wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Strychnine total synthesis wikipedia , lookup
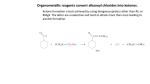
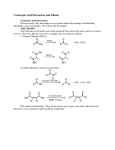
C AR BO XY LI C ACIDS a nd the ir Der iv ative s –N ucle op hilic Acyl su bstitut io n - Re view t he no me n clatu re fo r t hese co mp ou nd s in you r text b oo k - •• R Z •• R O O O R R Cl O R R OH R carboxylic acids acid chloride O < ~ anhydrides OR R esters NR'2 amides (R' = H or alkyl) the order of reactivity is the reverse (the better the leaving group, the more reactive RCOZ is in nucleophilic acyl substitution): O O O O Cl O > > R O R R OH carboxylic acids acid chloride R O > ~ anhydrides - Z O < < R R Z the basicity of Z determines the relative stability of carboxylic acid derivatives; thus, the order of stability is: O - O– O– O OR esters R NR'2 amides (R' = H or alkyl) based on this order of reactivity, more reactive acyl compounds (acid chlorides and anhydrides) can be converted to the less reactive ones (carboxylic acids, esters, and amides) but the reverse is usually not true. Why? O R O Z Nu R C Nu Z For a reaction to occur, Z must be a better leaving group than Nu 2 possible leaving groups For mat ion o f Acid C h lo rides Acid chlorides (or bromides) are extremely reactive. However, they are easily prepared from carboxylic acids by two common methods: 1) Treatment with thionyl Chloride (SOCl2): 1 - the poor leaving group, OH, is transformed into a better leaving group in this process- see M echa nis m 22 .5 . 2) Treatment with oxalyl chloride (a much nicer reaction!): cat. DMF This reaction is believed to proceed via the mechanism below: Why make acid chlorides? They are the fastest way to get to any other carboxylic acid derivative, or to a number of other carbonyl compounds: 2 The mechanism for all of the above reactions (except the last [Friedel-Crafts]) is pretty much the same – a nucleophile adds to the electrophilic carbonyl group, creating a tetrahedral intermediate. The electrons on oxygen then pop down, expelling the good leaving group (Cl-). This type of reaction is frequently called an addition-elimination reaction: Acid Anh ydr id es: O Ph O O O Ph benzoic anhydride Me O O Ph acetic benzoic anhydride Symmetrical anhydrides are typically made by mixing an acid under dehydrating conditions. For the most part, this is a difficult reaction to perform, and since the reactivity of anhydrides is so similar to that of acid chlorides, anhydrides are not commonly used in synthesis. 3 There are two exceptions to this statement. First, acetic anhydride is fairly easy to prepare in bulk from dirt-cheap acetic acid, and so it is often used in place of acetyl chloride (MeCOCl). Second, cyclic anhydrides are fairly easily formed by heating molecules that have two carboxylic acid functions in close proximity to high temperatures (a dehydration reaction). A couple of examples are maleic anhydride and phthalic anhydride: Cyclic anhydrides have use in organic chemistry; e.g. in Diels-Alder reactions. Again, the most commonly used anhydride is acetic anhydride. This reagent can be used to acetylate functional groups such as alcohols and amines. Acetylation can modify both the chemistry and biological activity of a compound. In the case of aspirin, for example, acetylation of the relatively acidic phenol alcohol of salicylic acid leads to a compound that doesn’t dissolve your stomach lining so easily... O NH2 Me HO H N O O Me Me O HO acetaminophen (active ingredient in tylenol) So, remember...anhydrides react just about like acid chlorides. 4 O R'OH as solvent O HCl or H2SO4 catalyst + H2O R OH R OR' -To drive the reaction to completion, excess alcohol must be used or water must be removed as it is formed. See Mechanism 22.6. - esterification of a carboxylic acid occurs in the presence of an acid but not in the presence of a base (since a carboxylate ion results under basic conditions). 5 performed on an ester rather than an acid. Finally, esters are very common in nature and thus are often the final goal of synthesis O R O 1. MeMgBr OH 2. H2O R + CH4 OH MeOH/H2SO4 O R OH 1. MeMgBr OMe 2. H2O R Me Me reverse of Fischer Esterification of carboxylic acids (see mechanism 22.8). 6 7 8 9 10 11