* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download CTLA-4
Monoclonal antibody wikipedia , lookup
Lymphopoiesis wikipedia , lookup
Immune system wikipedia , lookup
Gluten immunochemistry wikipedia , lookup
Polyclonal B cell response wikipedia , lookup
DNA vaccination wikipedia , lookup
Innate immune system wikipedia , lookup
Adaptive immune system wikipedia , lookup
Hygiene hypothesis wikipedia , lookup
Psychoneuroimmunology wikipedia , lookup
Adoptive cell transfer wikipedia , lookup
Sjögren syndrome wikipedia , lookup
Cancer immunotherapy wikipedia , lookup
Autoimmunity wikipedia , lookup
X-linked severe combined immunodeficiency wikipedia , lookup
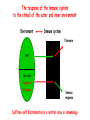
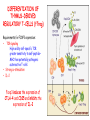
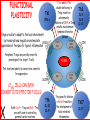
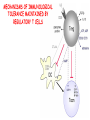
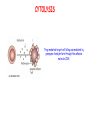
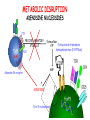
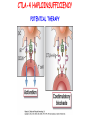
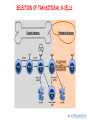
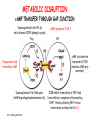
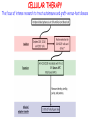
IMMUNOLOGICAL TOLERANCE ARPAD LANYI PhD [email protected] The response of the immune system to the stimuli of the outer and inner environment Environment Immune system Tolerance Self Non-self Dangerous Pathogenic Immune response Self/non-self discrimination is a central issue in immunology Immunological tolerance Definition: Lack of adaptive immune response against a given antigen. The interaction of the antigen with the lymphocytes induces unresponsiveness. ANTIGEN SPECIFIC!!! Unlike immunosuppression Why is this important? -All individuals are tolerant to their own antigens (self tolerance) -Failure of self tolerance results in autoimmunity -Terapeutic potential: Treat autoimmune diseases, allergic reaction or even tissue rejection Immunological tolerance CENTRAL TOLERANCE: Development of immunological tolerance begins in the primary (central) lymphoid organs (bone marrow and thymus) as an integral part of normal lymphocyte development PERIPHERAL TOLERANCE: Elimination/inhibition of autoreactive tolerance clones escaping central T-CELL TOLERANCE CENTRAL TOLERANCE OWING TO AIRE CENTRAL T-CELL TOLERANCE IS HIGHLY EFFECTIVE PERIPHERAL TOLERANCE Autoreactive T-cells encountering with self antigen in the peripheral tissues may be inactivated, deleted, or suppressed by regulatory T-cells In the absence of infection dendritic cells presenting self antigens do NOT express co-stimulatory molecules at high levels Antigen recognition in the absence of co-stimulation leads to a state of T-cell ANERGY ANERGY: FUNCTIONAL UNRESPONSIVENESS Phosphatases Ubiquitin ligases Binding of TCR to self antigens with high affinity in the absence of co-stimulation may lead to BIM-mediated apoptosis REPEATED stimulation of T-cells results in FAS-meditated apoptosis APOPTOSIS Bimmediated apoptosis Activationinduced cell death REGULATORY T LYMPHOCYTES EARLY EXPERIMENTAL MODELS SUGGESTING THE PRESENCE OF REGULATORY T-CELLS Murine neonatal thymectomy at day 3 leads to destruction of ovary (autoimmune) This phenotype can be prevented by injection of CD4+ T-cells/thymus transplantation Thymectomy did not cause autoimmunity if performed on day 1 or day 7 after birth Shortly after birth autoreactive T-cells leave the thymus, followed by suppressor T-cells a few days later, which can inhibit the activity of the autoreactive clones X IDENTIFICATION OF THE TREG LINEAGE AND ITS CELL SURFACE MARKERS BY DEPLETION EXPERIMENTS Nude (athymic) mice (no T-cells) + transfer normal CD4+ T-cells No autoimmunity Nude (athymic) mice (no T-cells) + transfer CD5-depleted CD4+ T-cells (antibody + complement) Autoimmunity Treg Teffector Sakaguchi et al. showed in Ann rev immun. 2004 stomach, thyroid, ovaries, testes X INHIBITION OF AUTOIMMUNE PROCESSES BY CD25+CD4+TR CELLS X FOXP3: MORE THAN A LINEAGE MARKER FOXP3 DEFICIENCY:IPEX Immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome Polyendocrinopathy: multiple disorders of the endocrine glands Type 1 diabetes mellitus (most common) develops within the first few months of life Autoimmune thyroid disease (hypo- or hyperthyroidism) Enteropathy: severe diarrhea, usually the first symptom, failure to thrive Dermatitis Autoimmune blood disorders are common; about half of affected individuals have anemia, thrombocytopenia, neutropenia IPEX syndrome may involve the liver and kidneys (tubular nephropathy) Most patients with IPEX are males and most of them die within the first 2 years of life without treatment (a few with a milder phenotype have survived into the second or third decade of life) Treatment: hematopoietic stem cell transplantation (HSCT) The function of CD4+FOXP3+CTLA-4+CD25+ regulatory T-cells is to suppress immune responses and maintain self-tolerance DIFFERENTIATION OF REGULATORY T-CELLS DIFFERENTIATION OF THYMUS-DERIVED REGULATORY T-CELLS (tTreg) Requirements for FOXP3 expression: • TCR signaling High avidity self-specific TCR: greater sensitivity to self-peptide– MHC than potentially pathogenic autoreactive T-cells • Strong co-stimulation • IL-2 Foxp3 induces the expression of CTLA-4 and CD25 and inhibits the expression of IL-2 Hassall’s corpuscles instruct dendritic cells to induce the development of regulatory T-cells in human thymus Watanabe et al. Nature 436, 1181 doi:10.1038/nature03886 DIFFERENTIATION OF PERIPHERAL REGULATORY TCELLS (pTreg) pTreg STAT5 FOXP3 Suboptimal TCR-activation Low level co-stimulation TGF-β, IL-2 Naive CD4 T-cell IL-12 IFN-γ IL-4 Th1 STAT1/4 T-bet Th2 STAT6 GATA-3 IL-6, IL-12, IL-23, TGF-β IL-1 IL-6 TGF-β Th17 STAT3 RORγT Tfh Bcl6 PARALLEL OR SEQUENTIAL DEVELOPMENT OF EFFECTOR AND REGULATORY T-CELLS The life of regulatory T cells Iris K. Gratz, Michael D. Rosenblum, and Abul K. Abbas Ann. N.Y. Acad. Sci. (2013) 1–5 THE UNEXPECTED BIOLOGY OF IL-2 Dual roles of IL-2 in T-cell responses Induction of immune response Control of immune response Prediction: What will be the consequence of eliminating IL-2 or the IL-2 receptor? Surprising conclusion from IL-2 knock out mice: the non-redundant function of IL-2 is in controlling immune responses (generalized autoimmune disease) FUNCTIONAL PLASTICITY Th1 IFN-γ pTregs are able to adapt to the local environment by transcriptional regulation and mediate CXCR3 suppression of the specific type of inflammation T-bet and GATA3 double deficiency in Tregs results in autoimmunity. Ablation of IRF4 in Tregs results in autoimmune lymphoproliferative disease. Peripheral T-regs can partially mimic the phenotype of the target T-cells. X CXCR5 Tfh Cxcr5-/- Bcl6-/-Treg Both Treg and are inefficient in controlling germinal center reactions. IL-4 IL-5 IL-13 CCR4 FOXP3 This functional plasticity seems to be essential for suppression. pTREG CELLS CAN EVEN CONVERT INTO EFFECTOR T-CELLS! STAT6 IRF4 GATA3 STAT1 Tbet Th2 IL-21 IL-4 Bcl6 STAT3 RORγ Treg-specific ablation of Stat3 results in the development of fatal intestinal inflammation. CCR6 Th17 IL-17 SUMMARY: Treg DIFFERENTIATION tTreg pTreg High affinity TCR specific for self antigen Self or non-self antigen-specific TCR Co-stimulation Suboptimal TCR signal IL-2 Low level co-stimulation Control of systemic and tissue-specific autoimmunity IL-2, TGF-β Control of local tissuespecific autoimmunity MECHANISMS OF REGULATION MECHANISMS OF IMMUNOLOGICAL TOLERANCE MAINTAINED BY REGULATORY T CELLS INHIBITORY CYTOKINES TGF-β: Inhibits the proliferation and effector functions of T-cells. Suppresses the classical activation of macrophages, neutrophils and endothelial cells. Stimulates production of IgA antibodies by inducing B-cells to switch to this isotype. (IgA is the major antibody isotype required for mucosal immunity.) Promotes tissue repair after local inflammatory reactions (stimulate collagen synthesis and angiogenesis). Membrane-tethered TGF-β can also mediate suppression by Treg cells in a cellcell contact-dependent manner. TGF-β knock out mice: progressive wasting syndrome and death 2 weeks after birth. INHIBITORY CYTOKINES IL-10: Inhibits the production of IL-12 by activated dendritic cells and macrophages and cell surface expression of co-stimulators and class II MHC molecules. Inherited deficiencies of IL-10 (develops before 1 year of age). or IL-10 receptor: severe colitis Knockout mice lacking IL-10 either in all cells or only in regulatory T-cells also develop colitis. IL-10 is especially important for controlling inflammatory reactions in mucosal tissues, particularly in the gastrointestinal tract. The Epstein-Barr virus contains a gene homologous to human IL-10, and viral IL-10 has the same activities as the natural cytokine (evolution of the virus, survival advantage). CYTOLYSIS Treg-mediated target-cell killing was mediated by granzyme A and perforin through the adhesion molecule CD18. doi:10.1038/nri2343 METABOLIC DISRUPTION CYTOKINE DEPRIVATION Tregs express all three components of the highaffinity IL-2R—CD25, CD122, and CD132—and IL-2 is essential for Tregs homeostasis. Tregs may compete with FOXP3− T-cells for IL-2, consume it, and inhibit the proliferation of FOXP3−T-cells, resulting in a form of apoptosis dependent on the pro-apoptotic factor Bim. doi:10.1016/j.immuni.2009.04.010 METABOLIC DISRUPTION cAMP TRANSFER THROUGH GAP JUNCTION Downregulation of miR-142-3p which silences ADCY9 (adenylyl cyclase) Treg Tregs produce high intracellular cAMP. Downregulation of the Pde3b gene (cAMP degrading phosphodiesterase 3b) DOI: 10.1002/eji.201141578 cAMP upregulates CTLA-4. Teff cAMP facilitates the expression of ICER (inducible cAMP early repressor). ICER inhibits transcription of NFAT and forms inhibitory complexes with preexisting NFAT, thereby inhibiting NFAT-driven transcription, including that of IL-2. METABOLIC DISRUPTION ADENOSINE NUCLEOSIDES P2Y Teff cAMP PRO-INFLAMMATORY STIMULUS P2X Extracellular Ectonucleoside triphosphate ATP diphosphohydrolase (E-NTPDase) AMP Adenosine 2A receptor ADENOSINE Ecto-5’-nucleotidase TARGETING DENDRITIC CELLS CTLA-4 COMPETITION TRANS-ENDOCYTOSIS TARGETING DENDRITIC CELLS CTLA-4 CTLA-4 promotes nuclear localization of Foxo3 transcription factor, which suppresses expression of genes encoding IL-6 and TNF. IDO (indoleamine-2,3-dioxygenase) induction is also CTLA-4 dependent. IDO catalyses the degradation of the essential amino acid L-triptophan to N-formylkynurenine, the initial, rate-limiting step of tryptophan catabolism. Effector T-cells starved of tryptophan are unable to proliferate and go into G1 cell cycle arrest. Metabolites of tryptophan including kynurenine, quinolinic acid, and picolinic acid are toxic to CD8+ and CD4+ Th1 cells. TNF TNF doi:10.1038/ni.1818 CTLA-4 DEFICIENCY Deletion of CTLA-4 causes systemic autoimmunity in mice. CTLA-4 deficiency in Tregs alone is sufficient to cause fatal disease and maintenance of its expression in activated effector T-cells is insufficient to prevent this outcome. In humans, mutations of CTLA4 resulting in CTLA-4 haploinsufficiency cause a complex immune dysregulation syndrome. Many patients previously diagnosed with CVID (Common variable immunodeficiency) carry CTLA-4 mutation. CTLA-4 haploinsufficiency is characterized by infiltration of T-cells into the gut, lungs, bone marrow, central nervous system, kidneys, and possibly other organs. Enteropathy, hypogammaglobulinemia, granulomatous lymphocytic interstitial lung disease, respiratory infections, splenomegaly, thrombocytopenia, hemolytic anemia, lymphadenopathy, psoriasis, thyroiditis, arthritis X CTLA-4 HAPLOINSUFFICIENCY Duodenum MRI pelvic Bone marrow biopsy Lung MRI Cerebellum Cerebellum 10.1038/nm.3746 Tissue infiltration and lymphadenopathy in patients with CTLA4 mutations. (a,b) Duodenal biopsies from patient B.II.4 (a) and A.III.3 (b) stained for CD4. (c) Highresolution chest computed tomography scan of the lungs from patient E.II.3. Arrows point to granulomatous-lymphocytic infiltration in both lungs. (d) Pulmonary lymphoid fibrotic lesions stained for CD4 in pulmonary biopsy from patient E.II.3. (e) Magnetic resonance imaging (MRI) of the pelvic area of patient A.III.3 with two enlarged lymph nodes (arrows) measuring up to 4 cm. (f) Bone marrow biopsy from patient B.II.4 stained for CD4. (g) MRI of gadolinium-enhanced lesion (arrows) in the cerebellum of patient A.III.1. (h) Resected cerebellar lesion from patient A.III.1 stained for CD3. Scale bars, 50 μm (a,b,d,f,h), 20 mm (c) and 50 mm (e). CTLA-4 HAPLOINSUFFICIENCY Schubert etal. Nat. Med 2014 doi:10.1038/nm.3746 X CTLA-4 HAPLOINSUFFICIENCY POTENTIAL THERAPY SUMMARY: PERIPHERAL MECHANISMS OF T-CELL TOLERANCE B-CELL TOLERANCE CENTRAL TOLERANCE NEGATIVE SELECTION ~75% of immature B-cells have some affinity for self antigens Many tissue-specific antigens are not expressed in the bone marrow 30-40% of transitional B-cells leaving the bone marrow are still autoreactive PERIPHERAL MECHANISMS OF B-CELL TOLERANCE SELECTION OF TRANSITIONAL B-CELLS doi:10.3390/antib3010116 ESTABLISHING T-CELL TOLERANCE IS ESSENTIAL TO MAINTAIN PERIPHERAL B-CELL TOLERANCE AUTOANTIBODY PRODUCTION IS DEPENDENT ON THE AVAILABILITY OF AUTOREACTIVE T-CELLS In the absence of T-cell help autoreactive B-cells are retained in the T-cell zone and die by apoptosis LACK OF DANGER SIGNALS RECEPTOR BLOCKADE: HIGH ZONE TOLERANCE THANK YOU IDENTIFICATION OF THE TREG LINEAGE AND ITS CELL SURFACE MARKERS BY DEPLETION EXPERIMENTS Nude (athymic) mice (no T-cells) + transfer normal CD4+ T-cells No autoimmunity Nude (athymic) mice (no T-cells) + transfer CD5-depleted CD4+ T-cells (antibody + complement) Autoimmunity Treg Teffector Sakaguchi et al. showed in Ann rev immun. 2004 stomach, thyroid, ovaries, testes X INHIBITION OF AUTOIMMUNE PROCESSES BY CD25+CD4+TR CELLS X METABOLIC DISRUPTION cAMP TRANSFER THROUGH GAP JUNCTION Downregulation of miR-142-3p which silences ADCY9 (adenylyl cyclase) Treg Tregs produce high intracellular cAMP. Downregulation of the Pde3b gene (cAMP degrading phosphodiesterase 3b) DOI: 10.1002/eji.201141578 cAMP upregulates CTLA-4. Teff cAMP facilitates the expression of ICER (inducible cAMP early repressor). ICER inhibits transcription of NFAT and forms inhibitory complexes with preexisting NFAT, thereby inhibiting NFAT-driven transcription, including that of IL-2. REGULATORY T-CELL-BASED IMMUNOTHERAPY X LOSS OF REGULATION OF AUTOREACTIVE T-CELLS RESULTS IN AUTOIMMUNITY ) doi:10.1038/nri2889 PHARMACOTHERAPIES Rapamycin: mTOR inhibitor, exploits the PI3 kinase pathway to preferentially expand Tregs. Clinically, rapamycin increases the number of Tregs in lung and renal transplant patients IL-2/IL-2 monoclonal antibody complexes: • 10-fold Treg expansion, resistance to experimental autoimmune encephalomyelitis and islet allograft rejection in mice • Clininal trial (Phase 1): subcutaneous IL-2 to treat active chronic GVHD, daily low-dose IL-2 was well-tolerated and led to sustained Treg expansion with improvement in GVHD manifestations Off-cell effect • Pharmaceuticals that stimulate Tregs may also activate conventional T-cells • Phase I clinical trial of TGN1412 – a super-agonistic anti-CD28 antibody – caused massive cytokine storm and multi-organ dysfunction CELLULAR THERAPY The focus of intense research to treat autoimmune and graft-versus-host disease Donor APC CLINICAL APPLICATION OF TREG CELLS Treg deficit associates with autoimmune disease development Failure to control islet-specific conventional T-cells results in type 1 diabetes mellitus (DM1) Risk of DM1 increases with the loss of FOXP3-expressing Tregs Treg adoptive transfer to non-obese diabetic (NOD) mice can prevent the development of DM1 Clinical trial: DM1 children: autologous CD4+CD25highCD127−Treg infusion • Decrease in the requirement of exogenous insulin in all the patients after 2 weeks • 4–5 months after Tregs administration: Of the 10 patients treated with Tregs, 8 were still in clinical remission, with 2 patients out of insulin completely CLINICAL APPLICATION OF TREG CELLS Transplantation Hematopoietic stem cell transplantation (HSCT) • Graft versus host disease • Inflammation often causes tissue immunosuppressive pharmacotherapy damage despite routine post-HSCT • Ongoing clinical trials support the use of CD4+CD25+ Tregs to suppress GVHD Solid organ transplantation • Phase I/II clinical trials to evaluate the safety and feasibility of various types of cell therapy including expanded Tregs in living-donor kidney transplantation CLINICAL APPLICATION OF TREG CELLS FOXP3+ Tregs impede the development of effective tumour immunity A large number of CD4+CD25+FOXP3+ are present in tumours and draining lymph nodes in patients with cancer. Decreased ratios of CD8+T-cells to CD4+CD25+FOXP3+ Tregs in tumours correlate with poor prognosis. It has also been shown in numerous mouse models that depletion of Tregs enhances antitumour immune responses, leading to the eradication of tumours. Studies in humans have shown that tumour antigen-specific CD4+T-cells can expand in patients with cancer and healthy individuals following in vitro antigenic stimulation of peripheral CD4+T-cells isolated from the individual after depletion of CD4+CD25+Tcells.