* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Impaired Tricarboxylic Acid Cycle Activity in Mouse Livers Lacking
Basal metabolic rate wikipedia , lookup
Metabolic network modelling wikipedia , lookup
Lactate dehydrogenase wikipedia , lookup
Fatty acid synthesis wikipedia , lookup
Metabolomics wikipedia , lookup
Pharmacometabolomics wikipedia , lookup
Isotopic labeling wikipedia , lookup
Phosphorylation wikipedia , lookup
Fatty acid metabolism wikipedia , lookup
Blood sugar level wikipedia , lookup
Biochemistry wikipedia , lookup
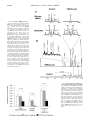
THE JOURNAL OF BIOLOGICAL CHEMISTRY Vol. 279, No. 47, Issue of November 19, pp. 48941–48949, 2004 Printed in U.S.A. Impaired Tricarboxylic Acid Cycle Activity in Mouse Livers Lacking Cytosolic Phosphoenolpyruvate Carboxykinase* Received for publication, June 24, 2004, and in revised form, August 30, 2004 Published, JBC Papers in Press, September 3, 2004, DOI 10.1074/jbc.M407120200 Shawn C. Burgess‡§, Natasha Hausler‡, Matthew Merritt‡, F. Mark H. Jeffrey‡, Charles Storey‡, Angela Milde‡, Seena Koshy¶, Jill Lindner储, Mark A. Magnuson储, Craig R. Malloy‡**, and A. Dean Sherry‡¶ From ‡The Mary Nell and Ralph B. Rogers Magnetic Resonance Center, Department of Radiology, University of Texas Southwestern Medical Center, Dallas, Texas 75235-9085, the ¶Department of Chemistry, University of Texas, Dallas, Texas 75083-0688, the 储Department of Molecular Physiology, Vanderbilt University School of Medicine, Nashville, Tennessee 37232-0615, and the **Department of Internal Medicine, Veterans Affairs North Texas Health Care System, University of Texas Southwestern Medical Center, Dallas, Texas 75235-9085 Liver-specific phosphoenolpyruvate carboxykinase (PEPCK) null mice, when fasted, maintain normal whole body glucose kinetics but develop dramatic hepatic steatosis. To identify the abnormalities of hepatic energy generation that lead to steatosis during fasting, we studied metabolic fluxes in livers lacking hepatic cytosolic PEPCK by NMR using 2H and 13C tracers. After a 4-h fast, glucose production from glycogenolysis and conversion of glycerol to glucose remains normal, whereas gluconeogenesis from tricarboxylic acid (TCA) cycle intermediates was nearly absent. Upon an extended 24-h fast, livers that lack PEPCK exhibit both 2-fold lower glucose production and oxygen consumption, compared with the controls, with all glucose production being derived only from glycerol. The mitochondrial reductionoxidation (red-ox) state, as indicated by the NADH/ NADⴙ ratio, is 5-fold higher, and hepatic TCA cycle intermediate concentrations are dramatically increased in the PEPCK null livers. Consistent with this, flux through the TCA cycle and pyruvate cycling pathways is 10- and 40-fold lower, respectively. Disruption of hepatic cataplerosis due to loss of PEPCK leads to the accumulation of TCA cycle intermediates and a nearly complete blockage of gluconeogenesis from amino acids and lactate (an energy demanding process) but intact gluconeogenesis from glycerol (which contributes to net NADH production). Inhibition of the TCA cycle and fatty acid oxidation due to increased TCA cycle intermediate concentrations and reduced mitochondrial red-ox state lead to the development of steatosis. Hepatic phosphoenolpyruvate carboxykinase (PEPCK)1 is a major control point for gluconeogenesis (1). Excess PEPCK * This work was supported in part by National Institutes of Health Grants RR02584, U24-DK59632, and HL-34557 and a grant from the American Diabetes Association (to M. A. M.). The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked “advertisement” in accordance with 18 U.S.C. Section 1734 solely to indicate this fact. § To whom correspondence should be addressed: the University of Texas Southwestern Medical Center, Mary Nell and Ralph B. Rogers Magnetic Resonance Center, 5801 Forest Park Rd., Dallas, TX 752359085. Tel.: 214-648-5893; Fax: 214-648-5881; E-mail: shawn.burgess@ utsouthwestern.edu. 1 The abbreviations used are: PEPCK, phosphoenolpyruvate carboxykinase; TCA, tricarboxylic acid; MAG, monoacetone glucose; PCA, perchloric acid; ACAC, acetoacetate; BHB, -hydroxybutyrate; OAA, oxaloacetate; PEP, phosphoenolpyruvate; red-ox, reduction-oxidation state. This paper is available on line at http://www.jbc.org expression in mice causes hyperglycemia (2), hyperinsulinemia, and increased glucose turnover (3). Inhibition of PEPCK by pharmaceutical interventions causes hypoglycemia (4) and, as expected, the global ablation of the cytosolic isoform of PEPCK in mice by genetic manipulation results in nonviable offspring (5). Most surprisingly, the liver-specific deletion of cytosolic PEPCK yielded a phenotype that, except during fasting and exercise, was virtually indistinguishable from control mice (5, 6). Even after a 24-h fast, when liver glycogen is depleted and flux through liver PEPCK should be essential to maintain plasma glucose, these animals are euglycemic and glucose turnover is normal. By using NMR spectroscopy and stable isotope tracers, we demonstrated that approximately ⬃60% of whole body glucose production in liver-specific PEPCK knock-out animals is derived from lactate and alanine (6). This suggests that either an alternative route to glucose production that bypasses PEPCK in these livers exists or that the majority of whole body gluconeogenesis is extrahepatic (6). In marked contrast to the minimal impact that the absence of hepatic PEPCK has on systemic glucose kinetics, these mice develop dramatic hepatic steatosis after fasting (6) even though enzymes of the TCA cycle and -oxidation are up-regulated (5) in liver tissue. This observation suggests that cataplerosis from the TCA cycle is tightly linked to oxidation of substrate in the TCA cycle (7). The purpose of the present work was to clarify the means by which PEPCK null livers contribute to glucose production and to determine how diminished PEPCK activity affects hepatic energy homeostasis. Although the lipid accumulation must be due to an imbalance of fatty acid uptake, -oxidation (8, 9), lipogenesis (10), or lipoprotein export (11), we sought to determine exactly how these processes are disturbed. Ideally, such investigations would be done in vivo, but such studies are complicated by the fact that other organs are known to contribute to gluconeogenesis in these animals (6). Thus, an isolated perfused liver preparation was chosen for these experiments to remove the influence of other organs and neurohumoral signals from liver function. As previous results (5) indicated a dramatic difference in metabolism depending on nutritional state, we chose to compare metabolism in livers from mice after a 24-h fast versus a short term 4-h fast. Here we used NMR and a combination of 2H and 13C tracers, isotopomer analysis, and metabolite assays to investigate the various pathways summarized in Fig. 1. The data provide evidence for near complete impairment of gluconeogenesis from TCA cycle cataplerosis, a reduced mitochondrial red-ox state, build-up of certain TCA cycle intermediates, and a 48941 48942 NMR Analysis of Livers Lacking PEPCK FIG. 1. Hepatic glucose metabolism. Pathways in boldface are interrogated by combined deuterium and 13C NMR isotopomer analysis. The deuterated water method introduces deuterium label into all the positions of glucose, but enrichment at H2, H5, and H6S occur at phosphoglucose isomerase, triose-phosphate isomerase, and fumarase making those positions diagnostic of glycogenolysis, GNGglycerol, and GNGPEP. [U-13C3]Propionate is used as vehicle to introduce 13C label into the TCA cycle intermediate pool by its conversion to succinyl-CoA. The resulting 13C isotopomers that form in either glucose or tissue glutamate are used to determine anaplerosis (assumed equal to cataplerosis, PEPCK), pyruvate cycling, and GNGPEP relative to TCA cycle turnover (citrate synthase). Pyruvate carboxylase and PEPCK are presumed to be the main sites of anaplerosis and cataplerosis, respectively. Note that the pyruvate cycling pathway via pyruvate kinase activity cannot be distinguished from pyruvate cycling due to malic enzyme activity. Substrates in boxes were supplied in the perfusate to the isolated perfused livers. dramatic inhibition of TCA cycle activity in the liver of PEPCK null mice. MATERIALS AND METHODS Chemicals—[U-13C3]Propionate (99%) and 2H2O (99%) were purchased from Cambridge Isotopes (Andover, MA). DSC-18 solid phase extraction gel and other common chemicals were purchased from Sigma unless otherwise noted. Animals—Liver-specific PEPCK knock-out mice, pcklox/lox/AlbCre (PEPCK null), and littermate controls, pcklox/lox (control), were generated as described previously (5). 3–5-Month-old mice weighing 25–30 g were maintained on standard laboratory chow. Prior to removal of livers for perfusion, animals were either fasted for 24 h (long term or LTfasted) or fasted for 12 h, fed a liquid diet of EnsureTM for 2 h, followed by a 4-h fast (short term or STfasted). Mice were fed in this manner to synchronize nutritional status by ensuring a 2-h feeding period, followed by a standard fast. The liquid diet also minimizes residual stomach contents.2 Liver Perfusion Experiments—All protocols were approved by the Institutional Animal Care and Use Committee. Animals were anesthetized by intramuscular injection of ketamine (Ketaset, Aveco, Fort Dodge, IA), and the livers were isolated and perfused as described previously (12), but with some modifications. Briefly, a mid-line laparotomy was performed to expose the liver and portal circulatory system. The portal vein was cannulated, and heparin (50 IU) was injected into the portal vein to prevent the formation of blood clots. The hepatic vein and inferior vena cava were dissected, and the perfusate flow through the portal vein was started simultaneously with a peristaltic pump at 8 ml/min. The liver was carefully removed from the carcass and suspended in a container of effluent perfusate, which was not re-circulated but constantly siphoned off and stored on ice for the duration of the experiment. The perfusate was composed of Krebs-Henseleit bicarbonate buffer containing 1.5 mM lactate, 0.15 mM pyruvate, 0.25 mM glycerol, 0.2 mM octanoate, 0.5 mM [U-13C3]propionate, and 3% v/v D2O. The livers were perfused for 60 min providing a total of 480 ml of perfusate. For a few livers, perfusions were continued for 2 h to confirm that isotopic steady state had been reached. After perfusion, livers were freeze-clamped using aluminum tongs cooled to ⫺80 °C and stored in a ⫺80 °C freezer until use. Fractions of perfusate were collected every 15 min for the assay of glucose production and oxygen consumption. pO2 was determined on afferent and efferent perfusate by using a blood gas analyzer (Instrumentation Laboratory, Lexington, MA), and oxygen consumption was calculated as detailed previously (13). Glucose was assayed by enzymecoupled reactions (14). Sample Preparation—After completion of the experiment, the perfusate was frozen until it was worked up completely. The perfusate was 2 C. Storey, A. Milde, and S. C. Burgess, unpublished observations. evaporated under vacuum at 50 °C. The resulting material was resuspended in 10 ml of 90:10 methanol/water and stirred for 10 min. The solution was decanted, and the step was repeated three times with all fractions combined and dried by evaporation under vacuum. The resulting partially de-salted material was dissolved in a minimum volume of water (about 5 ml) and passed through a column containing 20 ml of Amberlite IRA-67 anion exchange and 20 ml of Dowex 50W-X8-200 cation exchange resins (prepared as described in the product literature) in series. The glucose was removed with 60 ml of water; the pH was adjusted to 6 –7, and the sample was lyophilized and stored until further workup. Amino acids were isolated from some perfusate samples (15) to investigate alternative carbon disposal by the livers. NMR Analysis—Purified glucose was converted to the 1,2-isopropylidene glucofuranose derivative (monoacetone glucose (MAG)) as described previously (16, 17). The MAG samples were dissolved in 160 l of high pressure liquid chromatography grade acetonitrile with 5–10 l of water and transferred to a 3-mm NMR tube. Deuterium NMR spectra of MAG were collected using a 14.1-tesla Varian INOVA spectrometer and 3-mm broadband probe, tuned to 92-MHz as described previously (17, 18). A 90° pulse was applied, and the signal was acquired over 1 s (sweep width ⫽ 1000 Hz) with no further delay. 2H NMR spectra were typically signal-averaged for 4 h at 50 °C. 40 l of deuterated acetonitrile was added to the NMR sample for locking purposes, and 13C NMR spectra of MAG were collected as described previously (17) on the same spectrometer and probe tuned to 150-MHz. A 50° pulse, acquisition time of 1.5 s, and no further delay times were found to give the highest sensitivity for the MAG carbons (19). 13C NMR spectra of PCA extracts of the liver were recorded using a 45° pulse, 1.5-s acquisition time, and 1.5-s delay. Broad band 1H decoupling was accomplished using the standard WALTZ-16 technique. 2H and 13C spectra were analyzed using the curve-fitting routine supplied with NUTS PC-based NMR spectral analysis program (Acorn NMR Inc., Freemont, CA). Metabolic Profile—During the deuterated water experiment, the hydrogens attached to the carbon backbone of newly released glucose become enriched with 2H at specific locations that depend upon the synthetic pathway (Fig. 1). 2H enrichment of MAG positions H2, H5, and H6S (as evaluated by 2H NMR) were used to determine the origin of glucose. The 1-H2/H5 ratio, has been shown to represent the fraction of glucose originating from glycogenolysis (20, 21). Additionally, enrichment at the H6S (H6S/H2) position reflects gluconeogenesis from carbon units originating from the TCA cycle (oxaloacetate pool) (22, 23). The difference in the H5 and H6S enrichment ([H5-H6]/H2) reflects gluconeogenesis from substrates feeding the gluconeogenic pathway above the TCA cycle (presumably glycerol) (17, 19). Pathways intersecting the TCA cycle were evaluated by 13C isotopomer analysis of glucose (i.e. MAG) or glutamate (24). The 13C NMR multiplets in glucose or glutamate generated by the tracer [U-13C3]propionate were evaluated to determine flux (relative to TCA cycle flux) through anaplerosis/cataplerosis and pyruvate cycling (Fig. 1). Together the 2H and 13C data were integrated with total glucose NMR Analysis of Livers Lacking PEPCK 48943 FIG. 2. Oxygen consumption in isolated perfused null and control livers. Oxygen consumption was higher after a long term fast (24 h) than after a short term fast (4 h) in both the control and PEPCK null livers. PEPCK null livers had lower oxygen consumption than the control livers under either nutritional state, indicating a lower energy demand. Data are reported as the mean ⫾ S.D. for n ⫽ 4 –5 livers. production to determine TCA cycle flux (i.e. citrate synthase activity) as described previously (17, 19). Liver Tissue Analysis—Frozen liver samples were pulverized to a fine powder in a mortar and pestle cooled with liquid N2. The powder was agitated in 5 ml of ice cold 3% PCA in a 20-ml centrifuge tube. The suspension was centrifuged at 13,000 ⫻ g for 10 min at 4 °C. The liquid was decanted, and the plug was rinsed with a small volume of PCA that was combined with the decanted portion. The extract was neutralized to pH 7 with KOH, centrifuged at 13,000 ⫻ g for 10 min, decanted, and then lyophilized. The extract was dissolved in 600 l of 2H2O for 13C NMR isotopomer analysis of liver glutamate (24). The spectra of the control and null livers were also used to qualitatively observe the pool sizes of various metabolites that become 13C-enriched as a consequence of [U-13C3]propionate metabolism. About 100 mg of the liver tissue was saved for determination of mitochondrial red-ox state by analysis of the red-ox pair acetoacetate (ACAC) and -hydroxybutyrate (BHB) (25). The tissue was extracted, as above, with 600 l of 3% PCA. After centrifugation, the extract was neutralized with 80 l of concentrated KH2PO4. The samples were kept on ice, and ACAC and BHB were immediately evaluated by 1H NMR (26). 400 l of the extract was transferred to a 5-mm NMR tube, and an additional 100 l of a 0.5 mM sodium 2,2-dimethyl-2-silapentane-5sulfonate standard in 99% 2H2O was added. 1024 transients were collected with a 90° pulse width and 3-s acquisition time. Concentrations were determined with external standards of BHB referenced to the same internal 2,2-dimethyl-2-silapentane-5-sulfonate standard. With the sodium 2,2-dimethyl-2-silapentane-5-sulfonate resonance set to 0 ppm, the methyl resonances of BHB and ACAC had chemical shifts of 1.20 and 2.27 ppm, respectively. The limit of detection for this technique was about 4 M. The NAD⫹/NADH ratio was determined by assuming an equilibrium constant of 4.93 ⫻ 10⫺2 for the -hydroxybutyrate dehydrogenase reaction (25). Statistical Methods—All data are reported as the average ⫾ S.D. Statistical analysis of the data was performed using the two-tailed Student’s t test, assuming unequal variances. p values of less than 0.05 were considered significant. RESULTS A major advantage of using an isolated perfused mouse liver preparation is that tissue oxygen consumption and glucose production can be directly monitored as often as necessary. In experiments described below, oxygen consumption and glucose output were monitored every 15 min. The first 15-min perfusion period yielded sporadic values for oxygen consumption and glucose output, but the last 3 periods provided consistent results. Thus, glucose production and oxygen consumption from the last 3 periods (totaling 45 min) were averaged for each liver. Oxygen consumption (Fig. 2) was lower in livers from PEPCK null mice compared with littermate controls under both STfasted (controlled short term fast, see “Materials and Methods”) and LTfasted (long term fast, see “Materials and Methods”) conditions (p ⬍ 0.02, p ⬍ 0.03 respectively). Oxygen consumption was also lower in STfasted versus LTfasted livers from both null and control animals. Glucose Production—Glucose production was twice as high in control livers versus PEPCK null livers in LTfasted conditions and also tended to be higher in the STfasted condition (Fig. 3). In general, livers from STfasted mice had higher glucose output than livers from LTfasted mice regardless of genotype. Glucose production may have contributions from glycogen stores (i.e. glycogenolysis) or from gluconeogenesis. The carbon supply for gluconeogenesis can be provided from substrates such as glycerol that feed directly into the triose pool (GNGglycerol) or from substrates such as lactate, pyruvate, or amino acids that must undergo anaplerosis via pyruvate carboxylase to oxaloacetate (OAA) followed by cataplerosis via PEPCK to phosphoenolpyruvate (PEP) as the first steps of glucose synthesis (GNGPEP). The sources of glucose in this study were elucidated by using the deuterated water method (17, 18, 20, 23, 27). This technique most often uses mass spectrometry as the analytical tool but has been shown to work equally well when monitored by 2H NMR (18). Fig. 4 shows examples of 2H NMR spectra of MAG derived from glucose generated by livers of control and PEPCK null mice. The spectrum of MAG from the PEPCK null liver is clearly lacking substantial 2H in the H6 positions compared with the control. Because chemical exchange at the H6R and H6S occur at the level of alanine aminotransferase and fumarase, respectively (23), the low enrichment at these sites reflect severely limited gluconeogenesis supplied by cataplerotic flux from the TCA cycle. This is in agreement with the expected phenotype of a liver lacking PEPCK. These 2H NMR data were used to determine the contribution of PEP (formed from lactate or amino acids) to glucose production (i.e. GNGPEP). GNGPEP was lower by 11-fold in PEPCK null livers compared with controls under STfasted conditions (Fig. 3A) and 18-fold under LTfasted (Fig. 3B) conditions. Although gluconeogenesis due to cataplerosis from the TCA cycle is insignificant in PEPCK null livers, gluconeogenesis from glycerol (GNGglycerol) was maintained. Under STfasted conditions, GNGglycerol was significantly higher in control livers (0.61 mol/min/g) than in null livers (0.31 mol/min/g) (Fig. 3A). There was no significant difference in GNGglycerol under LTfasted conditions between control livers (0.54 mol/min/g) 48944 NMR Analysis of Livers Lacking PEPCK FIG. 3. Glucose metabolism in the isolated perfused livers of short term fasted (4 h, STfasted) (A) and long term fasted (B) (24 h, LTfasted) mice. Hepatic sources of glucose production (GP) were as determined by 2H NMR (as in Fig. 4) and glucose assay of effluent perfusate. Glycogenolysis (Gly) represents flux from glycogen to glucose. GNGglycerol represents flux from glycerol to glucose. GNGPEP represents flux from PEP (i.e. cataplerosis of TCA cycle intermediates via PEPCK) to glucose. As expected, GNGPEP is almost completely suppressed in the PEPCK null livers under both nutritional states. GNGglycerol did not supplement gluconeogenic capacity in the PEPCK null liver, and total glucose production was lower in the PEPCK null liver after the long term fast. Data are reported as the mean ⫾ S.D. for n ⫽ 4 –5 livers. and PEPCK null livers (0.37 mol/min/g) (Fig. 3B). Whereas GNGglycerol is less or equal in PEPCK null livers compared with controls, this source represented 92% of the glucose produced in the null liver compared with only 53% in the control livers. This result is in agreement with our earlier observation that the PEPCK null liver maintains its capacity to make glucose from glycerol (6). Glycogenolysis was low in livers of both PEPCK null and control mice in the LTfasted state (Fig. 3B). In contrast, PEPCK null and control livers showed substantial and equal rates of glycogenolysis, 0.69 and 0.73 mol/min/g, respectively, under STfasted conditions (Fig. 3A). Whereas absolute glycogenolysis was about the same in null and control livers, glycogen contributed a larger percentage of the glucose produced by null livers (68%) than control livers (49%) in the STfasted state. TCA Cycle Associated Pathways—Gluconeogenesis is intimately linked to the TCA cycle by both energy demand and substrate supply. To evaluate potential differences in TCA cycle fluxes, [U-13C3]propionate was used as a vehicle to deliver a 13C tracer into the TCA cycle via succinyl-CoA (28). The result is formation of multiple positional isomers of labeled intermediates commonly referred to as 13C isotopomers. The population of groups of 13C isotopomers is reflected by multi- plets due to spin-spin coupling between adjacent 13C nuclei (24) in all metabolites associated with the TCA cycle, and the relative areas of these multiplets is determined by the activity of pathways that intersect in the TCA cycle (e.g. citrate synthase, anaplerosis/cataplerosis, pyruvate cycling, and gluconeogenesis) (24). The combined information reported by the 2H and 13C NMR spectra plus absolute glucose production rates may then be used to determine absolute flux through PEPCK, pyruvate cycling (OAA 3 PEP 3 pyruvate 3 OAA or malate 3 pyruvate 3 OAA) and the TCA cycle (17, 19). Fluxes Relative to TCA Cycle Turnover—13C NMR spectra of MAG from effluent glucose synthesized by control and PEPCK null livers are shown in Fig. 5A. The C2 resonance of MAG from control livers showed multiplets typical of cataplerosis of TCA cycle intermediates into glucose. In contrast, the 13C spectrum of MAG derived from PEPCK null livers was almost devoid of multiplets, consistent with a lack of gluconeogenesis from TCA cycle intermediates. The low levels of multiplets in the C2 resonance of MAG from PEPCK null livers precluded a determination of anaplerotic/cataplerotic, gluconeogenic, and pyruvate cycling fluxes in the usual way (24). However, given that glucose and glutamate share the common TCA cycle intermediate, OAA, we turned our attention to analysis of the multiplets in the C2 resonance of liver glutamate (17, 24). This requires the assumption that a loss of cytosolic PEPCK activity has no impact on exchange of carbons between oxaloacetate and glutamate. The C2 resonances from the 13C NMR spectra of glutamate isolated from control and PEPCK null livers are also given in Fig. 5A. As anticipated, the C2 resonances of glutamate and glucose from control livers were similar in appearance, and an isotopomer analysis of each resonance gave identical measures of anaplerosis, gluconeogenesis, and pyruvate cycling flux (Fig. 6). This same analysis when applied to the C2 resonance of glutamate from PEPCK null livers indicated that anaplerosis and cataplerosis (relative to TCA cycle flux) were reduced by 3-fold and that pyruvate cycling flux (relative to TCA cycle flux) was suppressed ⬃10-fold in livers from LTfast animals (Fig. 6). Pyruvate cycling flux was reduced less (⬃4-fold) in livers after a STfast (not shown). By definition, anaplerosis must equal cataplerosis, whereas the difference between cataplerosis and pyruvate cycling is net output (Fig. 1). This output is normally assumed equal to gluconeogenesis because flux through PEPCK is considered the overwhelming site of cataplerosis in the liver (24). However, this is not true in PEPCK null mice, so output in this case could reflect some intermediate associated with the TCA cycle other than PEP. Fig. 5B shows the 13C NMR spectra of extracts of the control and PEPCK null livers. A comparison of the two spectra shows a dramatic increase in TCA cycle intermediate concentrations in PEPCK null livers compared with controls. The most dramatic increases are in malate, aspartate, glutamate, and fumarate, but citrate and succinate also appear to have increased (these changes were not quantitated). As summarized in Fig. 6, the glutamate isotopomer analysis reported that total cataplerosis (relative to TCA cycle flux) was lower by ⬃66% in livers from PEPCK null livers compared with controls. The residual cataplerosis of ⬃33% may be surprising given that GNGPEP is almost zero. The liver may also export amino acids (29), so effluent perfusate was fractionated into amino acids, organic acids, and neutral molecules by ion exchange chromatography to determine whether the increased tissue concentration of some intermediates might lead to increased export in the perfusate. 13C NMR and coupled enzymatic assay analysis of those fractions showed that 13C-labeled glutamine was exported nearly equally in PEPCK null and control LTfasted livers (6.7 and 9.0 nmol/min/g, respectively). Other labeled metabo- NMR Analysis of Livers Lacking PEPCK 48945 FIG. 4. Deuterium NMR spectra of MAG derived from glucose produced by LTfasted control (top) and PEPCK null (bottom) livers. Peak areas correspond to the relative deuterium enrichment of the seven hydrogen positions of glucose. H2, H5, and H6S enrichments are diagnostic of glycogenolysis (1-H5/H2), GNGglycerol ((H5-H6S)/H2)), and GNGPEP (1-(H5-H6S)/H2). The diagram shows how H6S enrichment occurs during the fumarase reaction in the TCA cycle but that those intermediates cannot enter the gluconeogenic pathways in the PEPCK null livers, resulting in low H6S enrichment in glucose produced from PEPCK null livers. Note that enrichment at the H6R position occurs during the pyruvate-alanine equilibrium catalyzed by alanine aminotransferase and is also very low in glucose from PEPCK null livers. lites were observed but in much lower concentrations. Thus, the combined export of molecules other than glucose cannot account for the cataplerotic flux ratio reported by the 13C NMR spectrum of glutamate from PEPCK null livers, because we would expect an efflux about 0.13 mol/min/g (33% of control GNGPEP according to Fig. 6). Absolute Rates of Flux—Alternatively, the anaplerotic flux ratio (anaplerosis/TCA cycle flux) as measured by NMR may not reflect a high anaplerotic and cataplerotic flux but rather low TCA cycle flux. This alternative explanation was tested by converting all relative flux values into absolute flux values by referencing them to glucose production (19). The absolute TCA cycle flux values derived from 13C NMR, 2H NMR, and glucose production data are compared in Fig. 7. TCA cycle flux estimated by the combined tracer experiments tended to be lower (p ⬍ 0.07) in LTfast controls (0.10 mol/min/g) versus STfast controls (0.18 mol/min/g), opposite the trend reported by oxygen consumption in these same groups (Fig. 2). Remarkably, TCA cycle flux in the PEPCK null livers was ⬃10-fold lower compared with controls in either nutritional state (Fig. 7). Although oxygen consumption was lower in PEPCK null livers in both STfast and LTfast states compared with controls, the magnitude of the difference was not as large as that measured for TCA cycle flux. Of the cataplerotic flux from OAA to PEP, a portion is converted to glucose (GNGPEP), and a portion is converted to pyruvate and then back into OAA by a process known as pyruvate cycling (see Fig. 1). By using a similar analysis as described above for TCA cycle flux, absolute pyruvate cycling flux was also determined. This flux was substantial in control livers after a STfast (0.43 mol/min/g) and after a LTfast (0.20 mol/min/g) but was greatly reduced in PEPCK null livers in both nutritional states (0.01 and 0.005 mol/min/g, respectively). The ⬃40-fold reduced pyruvate cycling flux in PEPCK null livers is consistent with impaired total cataplerosis (Fig. 6) and TCA cycle activity (Fig. 7) in livers lacking PEPCK. Mitochondrial Red-ox State—Perturbations of oxygen consumption, gluconeogenesis (30), -oxidation, lipogenesis (10), and intermediate pool sizes (31) could all be related to changes in the cellular red-ox state of liver from PEPCK null mice. Because the cytosolic red-ox state was presumably clamped by the 10:1 lactate/pyruvate supplied in the perfusate in these experiments (see “Materials and Methods”), mitochondrial red-ox was measured using the ACAC:BHB redox pair (25). Table I summarizes the tissue content of ACAC and BHB and the calculated mitochondrial NADH/NAD⫹ ratio. Remarkably, ACAC under LTfast conditions was reduced 5-fold in PEPCK null livers compared with controls, whereas BHB was unchanged, and this translates into a 5-fold increase in the NADH/NAD⫹ ratio in livers lacking PEPCK. DISCUSSION A global knock out of PEPCK in mice is lethal shortly after birth presumably because of insufficient glucose production from lactate/pyruvate/alanine during fasting (5). However, a liver-specific knock out of PEPCK is not lethal; such animals maintain euglycemia even after a 24-h fast apparently due to glucose production from other tissues (5, 6). Despite two prior studies that have explored the metabolic consequences resulting from the lack of PEPCK, many aspects of the phenotype of these mice are still a mystery. Indeed, these animals provide an experimental model that, when combined with the NMR tracer methodology, allows the consequences of altered hepatic energy balance to be further understood. In the current study, we explored the metabolic basis for the marked steatosis that occurs upon fasting in mice that lack hepatic PEPCK by using a preparation of isolated, perfused livers. We found that energy homeostasis is radically altered in livers lacking PEPCK as indicated by an abnormal mitochondrial hepatic red-ox state, increased TCA cycle intermediate pool sizes, reduced oxygen consumption, and a dramatically lower TCA cycle flux. These findings indicate that steatosis develops because of marked inhibition of fatty acid oxidation and that hepatic TCA cycle flux is an acutely sensitive interface for -oxidation and gluconeogenesis. Hepatic Glucose Production—PEPCK null livers maintain a relatively normal ability to produce glucose from glycogen and glycerol but have a severely limited ability to make glucose from TCA cycle cataplerosis. In the STfasted state, the difference in glucose production between PEPCK null and control livers did not differ significantly. Glycogenolysis was identical in PEPCK null and control livers in the STfasted state even though the glycogen content of PEPCK null livers has been reported to be lower (6). This could reflect an enhanced glycogen phosphorylase state in the PEPCK null liver that may be necessary to help maintain normal glucose output in the STfasted state. We 48946 NMR Analysis of Livers Lacking PEPCK FIG. 5. Carbon-13 NMR spectra. A, spectra of the C2 region of glucose-derived MAG from the perfusate effluent (top row) or liver glutamate (bottom row) from LTfasted control (left column) and PEPCK null (right column) livers. No multiplets are observed in the MAG C2 region of the PEPCK null sample because 13 C originates in the TCA cycle (via [U-13C3]propionate 3 succinyl-CoA). 13C NMR spectra of glutamate from liver extracts display multiplets in the C2 region of both the control (left column) and PEPCK null (right column) samples. Multiplets are labeled to identify the spinspin coupling responsible for each signal. For instance, D12 is the doublet present when C1 and C2 (but not C3) are 13Clabeled; D23 is the doublet present when C3 and C2 (but not C1) are 13C-labeled, and Q is the “quartet” present when C1, C2, and C3 are all 13C-labeled. The populations of these isotopomers are diagnostic of fluxes into and out of the TCA cycle and should yield the same results regardless of whether glucose C2 or glutamate C2 is analyzed because glucose carbons 1, 2, and 3 are equivalent to glutamate carbons 3, 2, and 1, respectively (assuming the same OAA pool is responsible for both). B, inspection of the entire 13C NMR spectra of the liver tissue extract reveals increased concentrations of TCA cycle intermediates in the PEPCK null livers compared with controls. FIG. 6. Relative flux of pathways intersecting the TCA cycle in fasted livers. Analysis of the C2 MAG or glutamate yields total anaplerosis from all pathways (assumed to be equal to total cataplerosis), pyruvate cycling (see Fig. 1), and output (the difference between cataplerosis and pyruvate cycling; usually considered gluconeogenesis). It is important to note that 13C NMR isotopomer analysis, by itself, gives flux ratios that are relative to TCA cycle turnover and not absolute flux. Sustained output or gluconeogenesis relative to the TCA cycle, despite the absence of PEPCK and GNGPEP, implies that absolute TCA cycle flux is decreased in the PEPCK null livers. Data are reported as the mean ⫾ S.D. for n ⫽ 4 –5 livers. NMR Analysis of Livers Lacking PEPCK 48947 FIG. 7. TCA cycle flux in control and PEPCK null livers determined from the combined 2H and 13C NMR data and absolute glucose production. PEPCK null livers had dramatically impaired TCA cycle flux under both nutritional conditions. Data are reported as the mean ⫾ S.D. for n ⫽ 4 –5 livers. TABLE I Mitochondrial redox state in fasted livers from control and PEPCK null mice Acetoacetate and -hydroxybutyrate concentrations (mol/g wet weight) were determined by 1H NMR of liver tissue extracts. NAD⫹/ NADH was determined from the acetoacetate/hydroxybutyrate ratio and an assumed equilibrium constant of 0.0493 for the BHB dehydrogenase reaction. Control PEPCK null ACAC BHB BHB/ACAC NAD⫹/NADH 0.19 ⫾ 0.07 0.055 ⫾ 0.005 0.19 ⫾ 0.06 0.18 ⫾ 0.08 1.0 5.1 20.1 4.0 showed previously (6) that PEPCK null livers perfused with 5 mM glycerol alone produce nearly four times as much glucose as control livers. However, in the present study, using more physiological levels of glycerol plus other substrates, glucose production from glycerol was either identical (LTfast) or lower (STfast) in PEPCK null livers compared with controls. This suggests that the livers of mice lacking PEPCK do not compensate by producing more glucose from glycerol. In the LTfasted state, glucose production is diminished by 50% in PEPCK null livers compared with controls (p ⬍ 0.05), in contrast to observations in vivo where glucose turnover did not differ between PEPCK null mice and littermate controls under LTfasted conditions (6). Glucose production in the LTfasted state is completely supported by GNGglycerol in PEPCK null livers, whereas the control livers have about an equal contribution from GNGglycerol and GNGPEP. This again is in contrast to the in vivo experiment where 60% of total body glucose was derived from GNGPEP in the liver-specific PEPCK null mice. This, together with the diminished glucose production in PEPCK null livers, supports the existence of an extrahepatic source of glucose production in the intact animal. Glyceroneogenesis and the PEPCK Null Liver—Glyceroneogenesis is often described as an “abbreviated” form of gluconeogenesis whereby the ultimate product is 3-glycerol phosphate rather than glucose (32). Just as in gluconeogenesis, PEPCK is a key regulatory enzyme in glyceroneogenesis. As reviewed by Reshef et al. (32), this process is necessary to support the triglyceride cycle, in which plasma free fatty acids are taken up by the liver and condensed with 3-glycerol phosphate to yield triglycerides. The importance of this cycle and its potential impact on hepatic cataplerosis are highlighted by reports that 75% of systemic free fatty acids are re-esterified and that the liver may account for most of this activity (32). Moreover, during fasting it is reported that a majority of 3-glycerol phosphate used for triglyceride synthesis in the liver is derived from glyceroneogenesis despite the presence of both exogenous glycerol and hepatic glycerol kinase (33). Thus, it is conceivable that a disruption in PEPCK activity might influence this cycle, resulting in perturbed hepatic lipid metabolism and steatosis. For instance, the role of PEPCK in glyceroneogenesis and the triglyceride cycle has been implicated as an important factor in the development of obesity and diabetes due to its influence on the storage and release of fatty acids (34). The evidence suggests that, in adipocytes, increased PEPCK activity leads to increased triglyceride storage and decreased free fatty acid release (due to increased triglyceride re-synthesis), whereas decreased activity leads to lipoatrophy and high plasma free fatty acid levels (due to decreased triglyceride re-synthesis) (34). Although glyceroneogenesis is clearly an important pathway in the liver, representing about 10% of the flux of gluconeogenesis (33), the effects of PEPCK expression on hepatic triglyceride metabolism seem less predictable than in the adipocyte. For example, mice overexpressing PEPCK 2-fold in the liver show little difference in plasma free fatty acid levels, plasma triglycerides, or hepatic triglycerides compared with littermate controls after a 6-h fast (3). Yet in the liver-specific PEPCK null mice studied here, there are dramatic increases in hepatic triglycerides (100%), plasma free fatty acids (60%), and plasma triglyceride levels (34%) after a 24-h fast (5). It has been suggested that altered hepatic lipid metabolism in the PEPCK null mice may stem from a loss of glyceroneogenesis and interruption of the triglyceride cycle (34, 35). Although increased triglyceride accumulation in the face of abolished PEPCK appears to be opposite the observations in adipocytes (34), the number of metabolic pathways that interact with anaplerosis and cataplerosis is much greater in the liver than in the adipocyte. The current data offer little to support or challenge the role of glyceroneogenesis and the triglyceride cycle in the development of hepatic steatosis in these animals but rather suggest that a substantial alteration in hepatic energy homeostasis may be responsible. Energy Homeostasis in the PEPCK Null Liver—Although the role of PEPCK in the intermediary metabolism of gluconeogenesis is well established, the data reported here clearly support a broader role of PEPCK in energy homeostasis via cataplerosis, which might include contributions to both gluconeogenesis and glyceroneogenesis. Livers lacking PEPCK display an abnormal energy homeostasis as follows: 1) a modest decrease in oxygen consumption, 2) an altered mitochondrial red-ox state, and 3) a 10-fold decrease in TCA cycle flux. The 5-fold increase in BHB/ACAC in PEPCK null livers reflects a 5-fold increase in the NADH/NAD⫹ ratio in the mitochondria of these livers. Similar changes in mitochondrial red-ox state have been implicated in other models of hepatic steatosis (36). A high level of mitochondrial NADH is known to inhibit -oxidation (8) and TCA cycle flux (37) and stimulate triacylglycerol formation (36). Overproduction of NADH may be related to an absence of 48948 NMR Analysis of Livers Lacking PEPCK PEPCK in three possible ways. First, the normally very active, NADH-consuming, glyceraldehyde-3-phosphate dehydrogenase reaction has little importance in the PEPCK null liver because PEPCK is not actively feeding triose units into gluconeogenesis. Second, the normal demand for ATP/GTP required to convert OAA into PEP is absent, so there is less energy demand to drive ATP synthesis in the PEPCK null liver. Third, gluconeogenesis from glycerol, which is maintained in the PEPCK null liver, generates excess cytosolic NADH, and this exacerbates the reduced energy needs of the PEPCK null liver. A direct consequence of a highly reduced mitochondrial red-ox state is that all NADH-producing steps in the TCA cycle are inhibited. This is reflected by the dramatically reduced TCA cycle flux observed here by using combined glucose production rates and 2H and 13C tracers. In addition to NADH/NAD, TCA cycle activity is also controlled by the concentration of cycle intermediates. For instance, citrate and succinyl-CoA are known to inhibit citrate synthase and ␣-ketoglutarate dehydrogenase (37), whereas oxaloacetate allosterically inhibits isocitrate dehydrogenase. Earlier we reported (5) a 10-fold increase in hepatic malate concentrations in these mice, and during the course of the current experiments we noted a similar increase in many other TCA cycle intermediates as detected by 13C NMR of the liver extracts (Fig. 5B). The impact of PEPCK on the concentration of TCA cycle intermediates is a direct reflection of its importance as a cataplerotic pathway. Under steady-state conditions anaplerosis and cataplerosis must always be balanced to maintain constant levels of TCA cycle intermediates (38). With the loss of PEPCK, the most important cataplerotic pathway in the liver, cycle intermediates accumulate because export of OAA is blocked. The resultant increase in TCA cycle intermediate concentration causes product and allosteric inhibition of cycle activity and fatty acid oxidation. One additional interesting observation was that the decrease in oxygen consumption in livers lacking PEPCK does not seem to parallel the reduction in TCA cycle activity. Oxygen consumption in the PEPCK null liver is certainly lower than control livers in both nutritional states but not to the extent predicted by the reduction in TCA cycle flux. However, it should be noted that ⬃20% of hepatic oxygen consumption may result from other oxygen-consuming reactions not linked to oxidative phosphorylation, as evidenced by the fact that hepatic oxygen consumption decreases only ⬃80% upon the addition of potassium cyanide to a perfused rat liver (39). Thus, one should not expect that the demand for residual oxygen consumption in a PEPCK null liver would be necessarily less than in control livers because a disproportionate amount of oxygen consumption in liver is due to reactions not linked to NADH disposal. Oxidative phosphorylation couples the transfer of electrons from NADH to oxygen with the associated production of ATP, but the NADH need not originate from the reactions of the TCA cycle. For example, -oxidation coupled to the export of ketones generates mitochondrial NADH without involving the reactions of the TCA cycle. Upon removal of all substrates from perfusion buffer of perfused rat livers, oxygen consumption falls only 33% (40), yet other parameters of metabolic activity can fall by as much as 80% of basal activity (41). Here we observe a 90 and 70% decrease in the TCA cycle activity in STfasted and LTfasted PEPCK null livers compared with controls, whereas oxygen consumption decreased by 50 and 20% respectively. Because ketone production in the perfused rat liver is known to vary between 0.17 and 0.60 mol/min/g (converted from dry weight in Ref. 42), under conditions similar to those used here, we anticipate that oxygen consumption reflects a combination of TCA cycle activity and ketogenesis. Although decreased oxygen consumption indicates that both lipid oxidation and TCA cycle flux is impaired in the PEPCK null livers, TCA cycle flux appears to be exquisitely sensitive to impaired gluconeogenesis. These data suggest that hepatic steatosis in the PEPCK null liver results from decreased lipid oxidation rather than increased de novo lipogenesis. This is also supported by the observation that pyruvate cycling is greatly reduced in the PEPCK null liver. Pyruvate cycling (Fig. 1) refers to any pathway involving combined carboxylation/decarboxylation of pyruvate. The two most common examples include pyruvate 3 OAA 3 PEP 3 pyruvate (PC ⫹ PEPCK ⫹ PK) or pyruvate 3 OAA 3 malate 3 pyruvate (PC ⫹ malate dehydrogenase ⫹ malic enzyme). The first pathway cannot exist in PEPCK null livers, but the second pathway could be of particular importance in these livers. Lipid biosynthesis in the liver involves transport of excess acetyl-CoA to the cytosol via citrate where it is cleaved by ATP-citrate lyase to acetyl-CoA and OAA. AcetylCoA can then be used for lipogenesis, and OAA must take a circuitous route back to the mitochondrial TCA cycle. OAA is converted first into malate (via malate dehydrogenase) and then pyruvate (via the malic enzyme), which then returns to the mitochondria before its conversion back to OAA by pyruvate carboxylase (37). This pathway links lipogenesis with pyruvate cycling and cannot be distinguished from the pyruvate cycling pathway involving PEPCK. Of the 14 NADPH molecules required for palmatate synthesis, 8 can be generated by the malic enzyme in converting excess malate into pyruvate (37). The fact that we see little pyruvate cycling activity in the PEPCK null liver suggests that lipogenesis is not an important mechanism in producing steatosis in these livers. One cannot, however, rule out participation of the pentose phosphate pathway in generation of NADPH for lipogenesis in these livers. Validation of the Deuterated Water Method—The deuterated water method for the analysis of gluconeogenesis takes advantage of hydrogen exchange at known enzymatic steps along the pathway toward glucose production. In its simplest form, the method uses the glucose H2/H5 deuterium enrichment ratio to evaluate glycogenolysis versus total gluconeogenesis as sources of glucose production (21, 23). The method has been used under a variety conditions, including diabetes and obesity (43) in children (44) and premature infants (45). Additionally, the 2H NMR method offers a convenient method to differentiate further the contributions of glycerol and TCA cycle intermediates to gluconeogenesis by detecting 2H enrichment at H6S (incorporated at the level of fumarase) versus H5 (incorporated at the level of triose-phosphate isomerase) (17, 19). This valuable method of quantitating glycerol contribution to gluconeogenesis has largely been overlooked, in part because its contribution is considered small in normal humans (17) and in rats (19, 46) but also because there has been no good way to validate the method. We now know that the contribution of glycerol to gluconeogenesis is actually quite high in the mouse (20 –30%) (6) and abnormally high in lipodystrophic patients on human immunodeficiency virus therapy (47). The PEPCK null liver offers a unique opportunity to validate the method because here the liver is largely incapable of generating glucose from TCA cycle intermediates. Indeed, glucose generated by these isolated livers has very little 2H enrichment at the H6 positions (Fig. 4), confirming for the first time that H5 and H6 are differentially enriched during the 2H2O experiment according to gluconeogenic contributions from glycerol and TCA cycle intermediates, respectively. Technical Considerations—The methodology employed here couples the deuterated water method with conventional 13C NMR Analysis of Livers Lacking PEPCK NMR isotopomer analysis. Most of the standard assumptions have already been detailed (17, 21, 23, 24), but it is worth mentioning a few here. First, in order to couple the TCA cycle and pyruvate cycle activities with glucose production, it is assumed that anaplerotic influx into the TCA cycle is equally balanced by a cataplerotic flux. Normally in liver, this cataplerotic flux is considered equal to gluconeogenic flux, but in livers lacking PEPCK, this might represent some other pathway. The only other 13C-enriched metabolites identified in the perfusate in these experiments were glutamine and lactate, but their amount was small and no different from the control livers. Second, the small (5%) contribution of PEP to glucose production in PEPCK null liver was estimated by measuring the areas of small H6R peaks in the 2H NMR spectrum (Fig. 4). This 5% estimate is consistent with the known mitochondrial PEPCK content of mouse liver (roughly 2%). Even though the error associated with measuring the areas of such small resonances can be large, we estimate that it translates into an error in our metabolic flux measurements of no more than 20%. Even with this level of error, it is clear that TCA cycle flux and pyruvate cycle flux are both significantly lower in livers lacking PEPCK. Conclusions—The metabolic consequences of eliminating PEPCK from liver are in many ways predictable, yet the impact of this genetic manipulation on energy homeostasis in liver was largely unanticipated. Livers lacking PEPCK continue to produce glucose from other substrates but during long term fasting cannot compensate by using glycerol as the sole gluconeogenic precursor. Removal of the energy-demanding PEPCK pathway results in an increase in mitochondrial red-ox state and dramatic inhibition of the TCA cycle. This results in reduced oxidation of fats via -oxidation, less production of ketones, and subsequent hepatic steatosis. The isolated perfused liver model also provided a unique opportunity to validate one aspect of the 2H NMR method, i.e. that 2H enrichment at H6 accurately reflects gluconeogenesis from the level of the TCA cycle. The combined 2H and 13C tracer methods verified that livers lacking PEPCK cannot generate glucose from TCA cycle precursors, provided an upper limit for flux from glycerol to glucose, and provided unique insights into metabolic relationships between TCA cycle flux and glucose production in liver. Acknowledgment—We thank Erin Smith for the excellent technical assistance. 10. 11. 12. 13. 14. 15. 16. 17. 18. 19. 20. 21. 22. 23. 24. 25. 26. 27. 28. 29. 30. 31. 32. 33. 34. 35. 36. 37. 38. REFERENCES 1. Hanson, R. W., and Reshef, L. (1997) Annu. Rev. Biochem. 66, 581– 611 2. Valera, A., Pujol, A., Pelegrin, M., and Bosch, F. (1994) Proc. Natl. Acad. Sci. U. S. A. 91, 9151–9154 3. Sun, Y., Liu, S., Ferguson, S., Wang, L., Klepcyk, P., Yun, J. S., and Friedman, J. E. (2002) J. Biol. Chem. 277, 23301–23307 4. DiTullio, N. W., Berkoff, C. E., Blank, B., Kostos, V., Stack, E. J., and Saunders, H. L. (1974) Biochem. J. 138, 387–394 5. She, P., Shiota, M., Shelton, K. D., Chalkley, R., Postic, C., and Magnuson, M. A. (2000) Mol. Cell. Biol. 20, 6508 – 6517 6. She, P., Burgess, S. C., Shiota, M., Flakoll, P., Donahue, E. P., Malloy, C. R., Sherry, A. D., and Magnuson, M. A. (2003) Diabetes 52, 1649 –1654 7. Magnuson, M. A., She, P., and Shiota, M. (2003) J. Biol. Chem. 278, 32485–32488 8. Bremer, J., and Wojtczak, A. B. (1972) Biochim. Biophys. Acta 280, 515–530 9. Geneve, J., Hayat-Bonan, B., Labbe, G., Degott, C., Letteron, P., Freneaux, E., 39. 40. 41. 42. 43. 44. 45. 46. 47. 48949 Le Dinh, T., Larrey, D., and Pessayre, D. (1987) J. Pharmacol. Exp. Ther. 242, 1133–1137 Fellenius, E., Bengtsson, G., and Kiessling, K. H. (1973) Acta Chem. Scand. 27, 2893–2901 Venkatesan, S., Ward, R. J., and Peters, T. J. (1988) Biochim. Biophys. Acta 960, 61– 66 Colet, J.-M., and Muller, R. N. (1994) Magn. Reson. Mat. Phys. Biol. Med. 2, 303–305 Neely, J. R., Liebermeister, H., Battersby, E. J., and Morgan, H. E. (1967) Am. J. Physiol. 212, 804 – 814 Kunst, A., Draeger, B., and Ziegenhorn, J. (1984) in Methods of Enzymatic Analysis (Bergmeyer, H. U., ed) 3rd Ed., pp. 163–172, VCH Verlagsgesllschaft, Weinheim, Germany Sherry, A. D., Zhao, P., Wiethoff, A. J., Jeffrey, F. M., and Malloy, C. R. (1998) Am. J. Physiol. 274, H591–H599 Burgess, S. C., Weis, B., Jones, J. G., Smith, E., Merritt, M. E., Margolis, D., Dean Sherry, A., and Malloy, C. R. (2003) Anal. Biochem. 312, 228 –234 Jones, J. G., Solomon, M. A., Cole, S. M., Sherry, A. D., and Malloy, C. R. (2001) Am. J. Physiol. 281, E848 –E856 Burgess, S. C., Nuss, M., Chandramouli, V., Hardin, D. S., Rice, M., Landau, B. R., Malloy, C. R., and Sherry, A. D. (2003) Anal. Biochem. 318, 321–324 Jin, E. S., Jones, J. G., Merritt, M., Burgess, S. C., Malloy, C. R., and Sherry, A. D. (2004) Anal. Biochem. 327, 149 –155 Schumann, W. C., Gastaldelli, A., Chandramouli, V., Previs, S. F., Pettiti, M., Ferrannini, E., and Landau, B. R. (2001) Anal. Biochem. 297, 195–197 Landau, B. R., Wahren, J., Chandramouli, V., Schumann, W. C., Ekberg, K., and Kalhan, S. C. (1996) J. Clin. Investig. 98, 378 –385 Kuwajima, M., Golden, S., Katz, J., Unger, R., Foster, D., and McGarry, J. (1986) J. Biol. Chem. 261, 2632–2637 Landau, B. R., Wahren, J., Chandramouli, V., Schumann, W. C., Ekberg, K., and Kalhan, S. C. (1995) J. Clin. Investig. 95, 172–178 Jones, J. G., Naidoo, R., Sherry, A. D., Jeffrey, F. M., Cottam, G. L., and Malloy, C. R. (1997) FEBS Lett. 412, 131–137 Williamson, D. H., Lund, P., and Krebs, H. A. (1967) Biochem. J. 103, 514 –527 Chung, Y., and Jue, T. (1992) Biochemistry 31, 11159 –11165 Wajngot, A., Chandramouli, V., Schumann, W. C., Ekberg, K., Jones, P. K., Efendic, S., and Landau, B. R. (2001) Metabolism 50, 47–52 Sherry, A. D., Sumegi, B., Miller, B., Cottam, G. L., Gavva, S., Jones, J. G., and Malloy, C. R. (1994) Biochemistry 33, 6268 – 6275 Cooper, A., Nieves, E., Rosenspire, K., Filc-DeRicco, S., Gelbard, A., and Brusilow, S. (1988) J. Biol. Chem. 263, 12268 –12273 Williamson, J. R., Browning, E. T., and Scholz, R. (1969) J. Biol. Chem. 244, 4607– 4616 Hiltunen, J. K., and Hassinen, I. E. (1977) Int. J. Biochem. 8, 505–509 Reshef, L., Olswang, Y., Cassuto, H., Blum, B., Croniger, C. M., Kalhan, S. C., Tilghman, S. M., and Hanson, R. W. (2003) J. Biol. Chem. 278, 30413–30416 Kalhan, S. C., Mahajan, S., Burkett, E., Reshef, L., and Hanson, R. W. (2001) J. Biol. Chem. 276, 12928 –12931 Beale, E. G., Hammer, R. E., Antoine, B., and Forest, C. (2004) Trends Endocrinol. Metab. 15, 129 –135 Beale, E. G., Hammer, R. E., Antoine, B., and Forest, C. (2002) FASEB J. 16, 1695–1696 Eaton, S., Record, C. O., and Bartlett, K. (1997) Eur. J. Clin. Investig. 27, 719 –722 Lehninger, A., Nelson, D., and Cox, M. (2000) in Principles of Biochemistry, (Nelson, D. L., and Cox, M. M., eds) 3rd Ed., pp. 586 –588, Worth Publishing, New York Owen, O. E., Kalhan, S. C., and Hanson, R. W. (2002) J. Biol. Chem. 277, 30409 –30412 Younes, M., and Strubelt, O. (1988) Pharmacol. Toxicol. 63, 382–385 Beauvieux, M.-C., Tissier, P., Gin, H., Canioni, P., and Gallis, J.-L. (2001) J. Nutr. 131, 1986 –1992 Dufour, S., Thiaudiere, E., Gallis, J.-L., Canioni, P., and Diolez, P. (1996) Magn. Reson. Mat. Phys. Biol. Med. 4, 329 Scholz, R., Schwabe, U., and Soboll, S. (1984) Eur. J. Biochem. 141, 223–230 Gastaldelli, A., Baldi, S., Pettiti, M., Toschi, E., Camastra, S., Natali, A., Landau, B. R., and Ferrannini, E. (2000) Diabetes 49, 1367–1373 Sunehag, A. L., Treuth, M. S., Toffolo, G., Butte, N. F., Cobelli, C., Bier, D. M., and Haymond, M. W. (2001) Pediatr. Res. 50, 115–123 Sunehag, A. L., Haymond, M. W., Schanler, R. J., Reeds, P. J., and Bier, D. M. (1999) Diabetes 48, 791– 800 Peroni, O., Large, V., and Beylot, M. (1995) Am. J. Physiol. 269, E516 –E523 Weis, B. C., Margolis, D., Burgess, S. C., Merritt, M. E., Wise, H., Sherry, A. D., and Malloy, C. R. (2004) Magn. Reson. Med. 51, 649 – 654