* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Amino Acids 2
Nucleic acid analogue wikipedia , lookup
Western blot wikipedia , lookup
Nuclear magnetic resonance spectroscopy of proteins wikipedia , lookup
Metalloprotein wikipedia , lookup
List of types of proteins wikipedia , lookup
Protein–protein interaction wikipedia , lookup
Intrinsically disordered proteins wikipedia , lookup
Protein folding wikipedia , lookup
Protein (nutrient) wikipedia , lookup
Ribosomally synthesized and post-translationally modified peptides wikipedia , lookup
Circular dichroism wikipedia , lookup
Self-assembling peptide wikipedia , lookup
Bottromycin wikipedia , lookup
Protein adsorption wikipedia , lookup
Genetic code wikipedia , lookup
Cell-penetrating peptide wikipedia , lookup
Peptide synthesis wikipedia , lookup
Expanded genetic code wikipedia , lookup
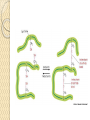
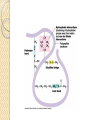
Amino Acids & Side Groups Polar Charged ◦ ACIDIC negatively charged amino acids ASP & GLU R group with a 2nd COOH that ionizes* above pH 7.0 Polar Charged ◦ BASIC positively charged amino acids LYS, ARG, HIS R group with a 2nd amide* that protonates below pH 7.0 Acidic Side Chains Basic Side Chains Acidic vs. Basic Polar Charged Amino Acids & Side Groups Polar Uncharged ◦ THR, TYR, ASN, GLN (cys) are soluble in water, i.e., hydrophilic (attract Hbonds) Contain hydroxyl or amino functional groups Polar Uncharged Hydroxyl Polar Uncharged II Amino Functional Groups Polar Uncharged Amino Acids Amino Acids & Side Groups NON-POLAR (aliphatic) ◦ Includes GLY, ALA, VAL, LEU, ILE, PRO all contain only hydrocarbons groups = hydrophobicity AROMATIC (hydrophobic non-polars) ◦ PHE & TRP (TYR) all contain R groups with Sulfur* R groups with sulfur ◦ MET, CYS ring structures* or Non-Polar Hydrocarbon R-Groups Non-Polar Aromatic R-Groups Non-Polar Sulfur R Groups Alpha Helix Beta-pleated sheets The most common polypeptide helix • Stabilized by extensive hydrogen bonding • Hydrogen bonds extend up from the oxygen from the carbonyl group to the NH group of a peptide linkage • ◦ This was shown in class via the visuals There are approximately 4 peptide bond links up stream between the atoms involved in the hydrogen bonds Each turn of an alpha helix contains 3.6 amino acids. • Unlike the alpha helix, composed of two or more peptide chains Polypeptide chains are joined by hydrogen bonds When the hydrogen bonds are formed between the polypeptide chains they are termed interchains. The polypeptide chains can run parallel to each other or antiparallel – Recall the “ends” of a polypeptide chain • C-terminus • N-terminus/Amino-terminus Secondary Protein Structures Alpha Helix Beta-pleated sheets Beta-pleated Sheets and Alzheimer’s Disease • • The amyloid protein, a class of fibrous proteins, is deposited in the brain. Individuals, that have Alzheimer’s Disease, have the amyloid protein composed of twisted Beta-pleated sheet fibrils whose three-dimentional structure is virtually identical to that of silk fibrils – Silk • Contain Beta-pleated sheet protein structures Tertiary Structure Interactions stabilizing Tertiary Structures ◦ Four were mentioned in class Disulfide Bonds Hydrophobic interactions Hydrogen bonds Ionic interactions Disulfide Bonds A disulfide bond is a covalent linkage formed by the sulfhydryl group (-SH) of two cysteine residues to form cystine • The folding of the polypeptide chain brings the cysteine residues near each other • Disulfide linkage contributes to the stability of the three-dimensional shape of the protein molecule • Disulfide bonds are found in proteins that are secreted by cells • – Thought that these strong covalent bonds help stabilize the structure of proteins and help prevent them from becoming denatured in the extra-cellular environment Hydrophobic Interactions • Recall that amino acids with non-polar side chains tend to be located in the interior of the polypeptide – Here, they associate with other hydrophobic amino acids • Special Note – Proteins located in non-polar (lipid) environments such as the phopholipid bilayer, tend to be in an opposite form • Hydrophobic amino acids are located on the surface • Hydrophilic amino acids are located on the interior Ionic Interactions Negatively charged groups interact with positively charged groups ◦ Negatively charged groups (-COO-) in the side chain of aspartate or glutamate ◦ Positively charged groups (-NH3+) in the side chain of lysine Dipole Moment Dipole Moment is the measure of a molecule’s overall polarity μ=Q*r ◦ μ = Dipole Moment ◦ Q = charge ◦ r = distance between charges Measured in debyes (\də-ˈbī\ ) Van der Waals Forces A weak attractive force between atoms or non-polar molecules caused by a temporary change in dipole moment ◦ Arising from a brief shift of orbital electrons to one side of one atom or molecule, creating a similar shift in adjacent atoms or molecules.