* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download What causes Earth`s climate and climate change?
Climate engineering wikipedia , lookup
Climate change mitigation wikipedia , lookup
Low-carbon economy wikipedia , lookup
Climate change and poverty wikipedia , lookup
General circulation model wikipedia , lookup
Climate sensitivity wikipedia , lookup
Scientific opinion on climate change wikipedia , lookup
Global warming controversy wikipedia , lookup
Surveys of scientists' views on climate change wikipedia , lookup
Climate change, industry and society wikipedia , lookup
Effects of global warming on Australia wikipedia , lookup
Fred Singer wikipedia , lookup
Physical impacts of climate change wikipedia , lookup
Public opinion on global warming wikipedia , lookup
Global warming hiatus wikipedia , lookup
Attribution of recent climate change wikipedia , lookup
Mitigation of global warming in Australia wikipedia , lookup
IPCC Fourth Assessment Report wikipedia , lookup
Global warming wikipedia , lookup
Politics of global warming wikipedia , lookup
Years of Living Dangerously wikipedia , lookup
Solar activity and climate wikipedia , lookup
Instrumental temperature record wikipedia , lookup
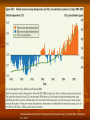
What causes Earth’s climate and climate change? The Atmosphere Earth’s atmosphere extends up to 500 km above ground 80% of air is concentrated in the first 16 km (troposphere) Weight of air 10,335 kg air m-2 land (or about 1 kg cm-2) Composition of air 78.1% N2; 20.9% O2; 0.93% Ar (argon) the rest, 0.07%, is water vapour, CO2 (carbon dioxide), CH4 (methane), O3 (ozone), and NO2 (nitrous oxide) CO2 = 389 ppm (parts per million, by volume) or 0.0389% only Air also contains suspended liquid water and solid particles (aerosols, typically 0.001 mm in diameter) Structure of atmosphere is stratified according to temperature Mount Everest (8,848 m) http://www.topnews.in/sports/teams/climbing E.A. Mathez, 2009, Climate Change: The Science of Global Warming and Our Energy Future, Columbia University Press. Source: Hartmann, 1994 Troposphere (lowest level, nearest to ground) up to (16 or 18) km: high in the tropics (equator), remains high then starts to fall at 40 N and S until about 8-10 km in the polar regions 80% of air is concentrated here and this layer is responsible for weather and contains the greenhouses gases Stratosphere up to 50 km contains mostly ozone which absorbs the deadly UV rays without UV absorption, life would not exist on Earth’s surface life would only exist several meters below surface only when ozone layer was developed, did life begin on Earth 400 mil. years ago Mesosphere up to 85 km Thermosphere up to 500 km very little air Troposphere Incoming solar radiation 50% absorbed by surface (ocean and ground) the rest is re-emitted as thermal energy and heats overlying air; thus, troposphere heated from bottom temperature decreases with increasing elevation decreasing by a mean of 6.5 C per km upward Troposphere has vigorous convection warm, low density air near ground rises cold, higher density air sinks to replace warm air causes weather due to these thermal motions but sometimes large mass of warm air rises and traps colder air below convection inhibited, trapping air pollutants in cities Stratosphere tropopause (about -70 C), but in stratosphere, air warms with increasing altitude because of ozone layer. Ozone absorbs UV; thus, heating air Mesosphere air temperature falls again with increasing altitude Thermosphere air temperature rises with increasing altitude because geomagnetic field interacts and absorbs some of the solar wind (high-speed streams of charged protons and electrons from the Sun; 400 km/s) during strong solar activity, temperatures may reach 1500 C, but we would not feel hot up there because of little atmosphere (gases) Little troposphere-stratosphere interaction mixing of the two layers is slow stratosphere is a stable layer danger if pollutants from ground reaches stratosphere pollutants tend to stay in the atmosphere for a long time and are quickly transported around the globe The stratified layers of the atmosphere is essential to life on Earth Cold tropopause traps water below because the cold causes condensation to ice particles in the troposphere if water enters into the upper layers, solar radiation would disassociate water molecule into H2 and O2 H2, being a very light gas, would not be held by gravity and be lost into space eventually increasingly more water would be lost, leaving Earth dehydrated no life on Earth this may have happened in our sister planet, Mars Earth rotates counter clockwise (eastward), looking down on North Hemisphere, but rotates clockwise, looking up on Southern Hemisphere http://sealevel.jpl.nasa.gov/overview/climate-earth.htm Coriolis Effect A < B rotational speed The Coriolis Effect causes things moving toward the poles to lead the earth's rotation because they are headed into regions where the earth's rotational speed is slower. They are deflected to the east. The Coriolis Effect causes things moving toward the equator to lag the earth's rotation because they are headed into regions where the earth's rotational speed is faster. They are deflected to the west. Idealized surface and global wind patterns E.A. Mathez, 2009, Climate Change: The Science of Global Warming and Our Energy Future, Columbia University Press. http://www.answers.com/topic/atmospheric-cell Global Atmospheric Circulation Solar heating imbalance means warm equatorial air flow towards the poles, and cold air from poles toward equator, creating a huge convection Hadley cell Warm air rises at the equator and flow downward to create two high pressure zones at 30 N and 30 S Some flow poleward, but most flows downward back to the equator, completing the Hadley cell Ferrel cell The Coriolis Effect breaks up Hadley cell into a second, mid-latitude circulation patterns, called Ferrel cell, which occur between 30 and 60 N and S less stable and give rise to a series of eddies flowing from west to east Polar cell occurs near the poles cold, dry air descends to form high-pressure vortices the downward air flows toward equator, but directed toward the west by Coriolis forces called polar easterlies polar easterlies meet the Ferrel cell along the polar front, creating a zone of unstable and severe weather Polar jet stream high-speed wind, hundreds of kilometers wide and several kilometers thick occurs at the polar front at about 10 km altitude undulates back and forth, and determines the weather between 45 and 60 N and S Subtropical jet stream occurs between 30 and 40 N and S, at 12 km altitude corresponds where the tropopause suddenly plunges to a lower elevation wind speeds up to 540 km h-1 but has less influence on weather in the lower latitudes than the polar jet stream at the higher latitudes E.A. Mathez, 2009, Climate Change: The Science of Global Warming and Our Energy Future, Columbia University Press. Source: Hartmann, 1994 Energy Budget (radioactive and non-radioactive transfers) http://asd-www.larc.nasa.gov/erbe/components2.gif Solar radiation spectrum Longer the wavelength, lesser the energy: PAR (photosynthetically active radiation, 400-700 nm, same as visible light) UV too high energy for plants NIR too low energy for plants Albedo of various surfaces Mean Earth’s surface albedo = 30% E.A. Mathez, 2009, Climate Change: The Science of Global Warming and Our Energy Future, Columbia University Press. Source: Hartmann, 1994 Surface Typical Albedo (%) Deep water, low wind, low latitude 7 Deep water, high wind, high latitude 12 Moist dark soil 10 Moist gray soil 15 Dry soil, desert 30 Wet sand 25 Dry light sand 35 Asphalt pavement 7 Concrete pavement 20 Short green vegetation 17 Dry vegetation 25 Coniferous forest 12 Deciduous forest 17 Forest with snow cover 25 Sea ice, no snow cover 30 Old, melting snow 50 Dry, cold snow 70 Fresh, dry snow 80 Greenhouse Effect Happens only in the troposphere GHGs transparent to incoming solar radiation but opaque to outgoing solar radiation; thus, entrapping heat www.eecs.umich.edu/mathscience/funexperiments/agesubject/lessons/images/diagrampa A passing electromagnetic radiation can "excite" a molecule, causing it to vibrate. Vibration modes of CO2: Mode (a) is symmetric and results in no net displacement of the molecule's "center of charge", and is therefore not associated with the absorption of radiation. Modes (b) and (c) not symmetrical and cause electrons not to be shared equally (thus, net charge) and these modes can absorb radiation, transferring the energy into to the molecule. GHG gases are molecules with 3 or more atoms N2 and O2 are not GHGs Image courtesy Martin C. Doege GHGs like CO2 and ozone (O3) are natural gases and are essential to life on Earth they help keep the Earth warm for life without GHG, Earth mean temperature would be -18 C, instead of 15 C GHG help to keep water in stable liquid form for life The problem today with GHG is too much of GHG is added into the atmosphere by human activities (anthropogenic) too much added warming caused by human activities like a person wearing too many thick clothes (heat cannot escape) A simple model with a non-absorbing atmosphere outgoing from Earth incoming from Sun Earth Total solar irradiance outside Earth (solar constant) is Fs = 1370 W m-2 The solar beams are parallel to Earth, so the power intercepted by Earth is contained in a tube of cross sectional area a2, where a is the Earth’s radius. The total solar energy received by Earth per unit time is thus Fsa2. Earth’s mean albedo, A, is 0.3, so total solar energy received, after reflection, is (1-A)Fsa2. If the Earth is a blackbody with uniform temperature, T, then by Stefan-Boltzman law, the power emiited per unit area is T4, where is the Stefan-Boltzman constant (5.6703 x 10-8 W m2 K4). On Earth, the power radiated is over all of Earth’s surface, where the total surface area is 4a2. So, the total power emitted from all of Earth’s surface area is 4a2T4. Assuming Earth is in thermal equilibrium, the incoming radiation must equal outgoing radiation to give (1-A)Fsa2 = 4a2T4 Substituting the values for A and Fs, T is about 255 K. But Earth’s measured surface temperature is about 288 K, higher than that calculated. Why? This is because this model does not include any greenhouse effect. A simple model of greenhouse effect F0 F0 Ta 1 1 A Fs 4 Fa lwFg ATMOSPHERE Fg Fg Tg4 swF0 Fa Tg GROUND Area of a2 has an irradiance of Fs, so the total surface area of Earth, 4a2, has an (lower) irradiance of F0, which is determined by a2 F0 1 A Fs 240 W m-2 2 4 a Of F0, swF0 is absorbed by the ground, and the remainder (1- sw)F0 is absorbed by the atmosphere. The ground emits Fg, where lwFg escapes the atmosphere, while the remainder, (1-lw)Fg, is absorbed by the atmosphere. Above the atmosphere, F0 Fa lw Fg Below atmosphere and ground, Fg Fa sw F0 Eliminating Fa, we obtain Fg Tg4 F0 1 sw 1 lw Taking rough estimates for sw and lw as 0.9 (strong transmittance, weak absorption) and 0.2 (weak transmittance, strong absorption), respectively, we obtain Tg (ground temperature) as 286 K (nearly the same as the mean measured value of 288 K) and Ta (atmosphere temperature) as 245 K. Recall: GHG has strong transmittance (high sw) for incoming solar radiation but strong absorption (low lw) for outgoing radiation GHG (Greenhouse gas) Water vapour (H2O) Contribution to greenhouse warming (%) GWP (Global warming potential for 100 years) Radiative forcing (RF) (W m-2) 55-70 Lifetime (years) 9 days Carbon dioxide (CO2) 25 1 1.66 50-200 days Chlorofluorocarbons (CFC) 11 10,900 0.34 100 Methane (CH4) 5 25 0.48 12 Nitrous oxide (NO2) 2 298 0.16 114 Ozone (O3) 1 0.35 GWP – measure of GHG potency in relation to CO2 RF – measure of warming/cooling caused by GHG GWPs are calculated as the ratio of the radiative forcing that would result from the emissions of one kilogram of a greenhouse gas to that from the emission of one kilogram of carbon dioxide over a period of time (usually 100 years) Radiative forcing (RF) is the additional radiative power that the gas is sending back to the ground values based on a base period (taken at year 1750, which is the start of the Industrial period) positive RF means increased heating (causes warming) negative RF means less heating (causes cooling) 1.66 0.34 0.16 0.48 1.6 0.35 0.07 0.01 0.1 0.12 -0.05 -0.2 -0.5 -0.7 IPCC, 2007 Contributions of different greenhouse gases to radiative forcing E.A. Mathez, 2009, Climate Change: The Science of Global Warming and Our Energy Future, Columbia University Press. Data from Forster et al., 2007 +1.7 ppm/year +0.6 ppb/year CO2 and NO2 continue to increase. Methane (CH4) and CFCs are levelling off. http://www.greencarcongress.com/2006/05/noaa_reports_st.html Other GHGs Halocarbons (hydrofluorocarbons, perfluorocarbons, and sulfur hexafluoride) from semiconductor industry Methyl bromide from fumigant for insect control in agriculture Nitrogen trifluoride (NF3) from production of flat LCD and plasma TVs Black soot caused by incomplete burning of fossil fuels black particles (aerosols) will absorb heat (causing warming) Model estimates of the direct effects of aerosols on radiative forcing Radiative forcing Aerosol (watts per square meter) Sulfate (sulfuric acid via sulfur dioxide from fossil-fuel burning) -0.4 ± 0.20 Organic carbon (from fossil-fuel and biomass burning) -0.05 ± 0.05 Black carbon (from incomplete fossil-fuel combustion) +0.20 ± 0.15 Smoke (compounds from forest fires) +0.03 ± 0.12 Nitrates (ammonium, from ammonia and NOx emissions) -0.10 ± 0.10 Anthropogenic dust (agriculture, cement production, drying soils) -0.1 ± 0.20 All aerosols combined, direct effects -0.50 ± 0.40 Increase in cloud albedo due to aerosols -0.3 to -1.8 E.A. Mathez, 2009, Climate Change: The Science of Global Warming and Our Energy Future, Columbia University Press. Source: Forster et al., 2007 Scanning electron microscope images of various aerosols E.A. Mathez, 2009, Climate Change: The Science of Global Warming and Our Energy Future, Columbia University Press. Photographs by V. Martins, NASA Global distribution of aerosols (2006) E.A. Mathez, 2009, Climate Change: The Science of Global Warming and Our Energy Future, Columbia University Press. Source: Reto Stöckli, NASA The Keeling Curve (CO2 levels from 1958 onward) May Oct The annual fluctuation in CO2 is caused by seasonal variations in CO2 uptake by land vegetation. Since more vegetation are concentrated in the Northern Hemisphere, more CO2 is removed from the atmosphere during Northern summer than Southern summer. http://en.wikipedia.org/wiki/File:Mauna_Loa_Carbon_Dioxide-en.svg CO2 levels 1750 (start of Industrialization) = 275 ppm today (2010) = 389 ppm increases about 1.7 ppm per year present CO2 levels are the highest in the last 400,000 years, based on ice core studies Since 1865, global mean surface temperature has increased from 14.4 to 15.4 C, the warmest in the last 1000 years temperature anomaly CO2 World development report 2010: Development and Climate Change, The World Bank, Washington D.C., 2010 Energy balance is imbalance by as much as +0.25 to 0.75 Wm-2 -2 mean: +0.5 Wm 90% of it is absorbed by the oceans “warming in the pipeline” even if there is no CO2 emissions today, the world will still warm by up to 0.6 C because the oceans will slowly release the heat into the atmosphere 0.75 0.25 C per Wm-2 forcing Ought to reduce CO2 levels back to 350 ppm, not 450 ppm, to stabilize the energy balance (bring it back to balance) Since start of Industrial Revolution = 275 ppm CO2 and in 2008, about 385 ppm CO2, and solar forcing by CO2 = 1.66 Wm-2 -2 Thus, (385-275)/1.66 = 66 ppm CO2 per Wm forcing Energy imbalance is 0.5 Wm-2, so to remove this imbalance, the level of CO2 must be reduce to 0.5 x 66 = 33 ppm CO2 must be removed to restore energy balance so, 385 - 33 = 352 ppm (about 350 ppm) is the level of CO2 to achieve Little doubt that human activities have caused global warming 90% certainty according to IPCC 2007 report The question is how much global warming will occur still uncertain because of feedback mechanisms where climate component A influences component B which, in turn, B influences A Feedback mechanisms on global warming Links between various elements of the climate system, illustrating how changes in any one can interact with others to produce positive or negative feedback effects Climate change : the science, impacts and solutions by A. Barrie Pittock, CSIRO, 2009 Examples of feedback: more CO2, higher temperature, which causes higher evaporation of water into the atmosphere (water is a GHG and more water vapour would amplify the greenhouse warming) => effect of CO2 is amplified increase in water vapor changes the vertical temperature distribution of the atmosphere close to ground, little effect by vapour because air already concentrated with vapour but higher up (8 km), air is much drier, and the addition of water vapour would amplify the greenhouse warming more vapour also means more clouds clouds trap heat (causing heating), but they also reflect solar radiation (causing cooling) industrial pollution of air may cause cooling by reflecting solar radiation back into space pollutant aerosols such as sulfates volcanic activity can also reduce warming by sulfur and ash emissions which reflect heat from solar radiation Mt. Krakatoa, Indonesia, erupted on 1883 reduced world temperature by 1.2 C for several years Mt. Pinatubo, Philippines, erupted on June 15, 1991 reduced world mean surface temperature by 1 C for two years Mt. El Chichon, Mexico, erupted on 28 March 1982 reduced world temperature by 0.3-0.5 C Mt. St. Helens, USA, erupted on May 18, 1980 reduced world temperature by 0.1 C http://wattsupwiththat.com/2009/01/13/how-did-the-el-chicon-and-pinatubo-volcanic-eruptions-affect-global-temperature/ Stratospheric aerosol before and on several occasions after the eruption of Mount Pinatubo, June 15, 1991 E.A. Mathez, 2009, Climate Change: The Science of Global Warming and Our Energy Future, Columbia University Press. Source: NASA Effect of clouds on warming Earth’s average albedo (including clouds) is 0.3; that is, 30% solar radiation is reflected clouds reflect incoming solar radiation (cooling) but clouds absorb emitted radiation from surface below (warming) absorbed heat is reemitted into space or back to the surface clouds act as insulator for surface low, thick cumulus clouds reflect more incoming radiation than they absorb (cooling) high, thin cirrus clouds transmit incoming radiation but trap some outgoing radiation (warming) overall: net effect of clouds on global warming is cooling, but much uncertainty still persist on cloud feedback mechanisms Cumulus Cirrus Cumulus cloud photo (left) by Carlye Calvin. Cirrus clouds photo (right) by Caspar Ammann.