* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download Introduction to spectroscopy
Franck–Condon principle wikipedia , lookup
Bremsstrahlung wikipedia , lookup
Chemical bond wikipedia , lookup
Metastable inner-shell molecular state wikipedia , lookup
Atomic absorption spectroscopy wikipedia , lookup
Resonance (chemistry) wikipedia , lookup
Computational chemistry wikipedia , lookup
Low-energy electron diffraction wikipedia , lookup
Analytical chemistry wikipedia , lookup
Rutherford backscattering spectrometry wikipedia , lookup
Biochemistry wikipedia , lookup
Metallic bonding wikipedia , lookup
Electron scattering wikipedia , lookup
Gamma spectroscopy wikipedia , lookup
Chemical imaging wikipedia , lookup
Rotational spectroscopy wikipedia , lookup
Electron configuration wikipedia , lookup
Nanochemistry wikipedia , lookup
History of molecular theory wikipedia , lookup
Wave–particle duality wikipedia , lookup
Physical organic chemistry wikipedia , lookup
Two-dimensional nuclear magnetic resonance spectroscopy wikipedia , lookup
Photosynthetic reaction centre wikipedia , lookup
Magnetic circular dichroism wikipedia , lookup
Mössbauer spectroscopy wikipedia , lookup
X-ray photoelectron spectroscopy wikipedia , lookup
Photoelectric effect wikipedia , lookup
Atomic theory wikipedia , lookup
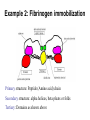
125:583 Biointerfacial Characterization Introduction to Spectroscopy Sep 28, 2006 Oct 12, 16, 2006 Yves Chabal Departments of Chemistry and Chemical Biology, and Biomedical Engineering Nanophysics Lab, Room 205 [email protected] Prabhas Moghe Departments of Chemical Engineering, and Biomedical Engineering LECTURE #1: Introduction Spectroscopy Spectrum: A plot of the intensity as a function light or particle energy (frequency, wavelength) Spectroscopy: Using a probe (radiation, ions or electrons) and sorting its content into energy bins to identify the materials response in each region of the spectrum Recall that any material system made up of atoms, molecules and electrons responds to external stimuli such as light or particles over a wide range of energies in a distinct manner Basics of Light, E&M Spectrum, and X-rays Light can take on many forms. Radio waves, microwaves, infrared, visible, ultraviolet, X-ray and gamma radiation are all different forms of light. The energy of the photon tells what kind of light it is. Radio waves are composed of low energy photons. Optical photons--the only photons perceived by the human eye--are a million times more energetic than the typical radio photon. The energies of X-ray photons range from hundreds to thousands of times higher than that of optical photons. The speed of the particles when they collide or vibrate sets a limit on the energy of the photon. The speed is also a measure of temperature. (On a hot day, the particles in the air are moving faster than on a cold day.) Very low temperatures (hundreds of degrees below zero Celsius) produce low energy radio and microwave photons, whereas cool bodies like ours (about 30 degrees Celsius) produce infrared radiation. Very high temperatures (millions of degrees Celsius) produce X-rays. Materials response to radiation or particles • E&M radiation interacts with materials because electrons and molecules in materials are polarizable: •(refraction, absorption) ñ= n+ i k n = refraction, k = absorption Atoms/molecules Valence electrons Core electrons • Ions, electrons and atoms incident on materials can interact with materials because they are either charged or can scatter from atomic cores Techniques and information content Molecular Molecular Libration vibrations (hindered rotations) Electronic Absorption Valence band and shallow electronic levels (atoms) Infrared, Raman, EELS Microwave, THz Deep electronic core levels (atoms) UV absorption UV photoemission Electron loss Visible Fluorescence Luminescence X-ray photoemission (XPS, ESCA) Auger Electron (AES) Photoelectron Spectroscopy Photons in Electrons out Vacuum level Valence electrons Core electrons • X-ray (photon) penetration into solid is large (~ microns) • Electron escape from solid is only from shallow region (~ 5-10 Å) because of short mean free path of electrons with energies between 10 and 1000 eV XPS is only sensitive to surface and near surface region Optical Spectroscopy Photons in Photons out • Large penetration into solid • Low energy photons Non destructive Photons • Can interact linearly (absorption) or non-linearly (Raman, harmonic generation) out FTIR Surface Spectroscopy • Infrared Spectroscopy Theory • IR spectrometers Grating systems Interferometers (FTIR) • Surface Spectroscopy Methods • Examples Classical theory for linear absorption • The electronic interactions between atoms in molecules or solids provide a binding force and a restoring force often compared to springs. Therefore each system (molecule, solid) displays characteristic vibrations (normal modes) associated with bond stretching and bond bending motions (just like a spring pendulum) • The frequency of the radiation identical to the frequency of these characteristic vibrations is absorbed • Absorption of infrared radiation by a vibrating molecule can only take place if the vibration produces an alternating electric field (changing dipole moment) e.g. O–C–O symmetric stretch (IR inactive) O–C–O asymmetric stretch (IR active) O–C–O bending mode (IR active) Examples Stretching modes -CH2- asym. stretching as(CH2) sym. stretching s(CH2) Bending modes -CH2- scissoring s(CH2) rocking (CH2) wagging (CH2) x twisting (CH2) LECTURE # 2: Instruments and surface spectroscopy October 12, 2006 Grating or prism spectrometer Source Selects one wavelength (energy) at a time, requiring rotation to scan the spectrum Array detectors allow detection of a restricted range of wavelengths Good to study single vibrational line (e.g. time resolved spectroscopy) Higher resolution requires narrowing slits Inefficient for high resolution spectroscopy Requires calibration Interferometers Detect IR intensity as a function of mirror displacement: INTERFEROGRAM Michelson Interferometer (broadband) http://www.wooster.edu/chemistry/is/brubaker/ir/ir_works_modern.html All wavelengths are measured simultaneously (Felgett advantage) Faster and more efficient No need for narrow slits (resolution determined by mirror travel) higher optical throughput (Jacquinot advantage) Internally calibrated by He-Ne laser control of moving mirror (Connes advantage) Ideal to examine broad spectral regions and weak absorptions with high resolution Fourier-Transform Infrared spectroscopy As more frequencies are added, the interferogram becomes a more complex function, with the largest amplitude at the zero path difference (zpd) For a single frequency (i.e. laser light), the signal on the detector (interferogram) is a sine wave Spectrum 25 Interferogram FT Waveforms Mirror displacement Absorbance 20 15 10 5 0 500 For a broad spectral range (white light), The interferogram is most peaked at zpd http://www.wooster.edu/chemistry/is/brubaker/ir/ir_works_modern.html 1000 1500 2000 2500 3000 3500 -1 Wavenumber (cm ) wavenumber ~ 1 f c 400 cm-1 - 4000 cm-1 25000 nm - 2500 nm Surface and Interface Spectroscopy IR wavelength (~ m) is much larger than surface dimensions (nm) Need to Eliminate all other contributions to spectrum (selecting a reference system) Final state Initial state (reference) 25 25 SiO2+Si 15 10 Si(111) 5 0 1000 1500 2000 2500 3000 15 10 Si(111) 5 0 etching 500 SiH+Si 20 Absorbance Absorbance 20 500 3500 1000 1500 2000 2500 3000 3500 -1 Wavenumber (cm ) -1 Wavenumber (cm ) 0.006 Reprocessing: Absorbance Subtraction of reference spectrum from final state spectrum SiH added 0.004 0.002 0.000 -0.002 SiO2 removed -0.004 -0.006 500 1000 1500 2000 2500 3000 -1 Wavenumber (cm ) 3500 4000 Maximizing Surface Interaction 1. For highly absorbing or reflecting (metal) substrates grazing incidence reflection tan (B) = ñ IR in IR out n and k large Reflection IR in 2. For weakly absorbing substrates “Brewster” incidence transmission tan (B) = n Need double-sided polish + bevels at sides In-situ possible for liquid environments k small IR out Transmission IR in 3. For transparent substrates Multiple internal reflections int ~ 45o int n large (2-4) k very small IR out Multiple internal Reflections Evanescent field ~ 1-10 m Attenuated Total Reflection (ATR) • Multiple internal reflection: IR in IR out • In-situ wet chemistry/electrochemistry contact IR in liquid out IR out liquid in electrodes • Multiple internal transmission: (Handbook of Vibrational Spectroscopy, Wiley, Vol.1, p. 1117, 2002) IR in IR out Buried interface LECTURE #3: Applications October 16, 2006 Example 1: FTIR for biointerfacial characterization Attaching linker for biomolecule (e.g. antibody) immobilization on Silicon substrate MPS models a tiny antibody! Step 2: Formation of Urea linkage during PMPI attachment Step 3: Formation of succinimide (evidence for thioether bonding) during MPS attachment Example 2: Fibrinogen immobilization Primary structure: Peptide (Amino acid) chain Secondary structure: alpha helices, beta pleats or folds Tertiary: Domains as shown above Fibrinogen structure and composition Hydrophobic Amino acids Primary structure: Peptide (Amino acid) chain Secondary structure: alpha helices, beta pleats or folds Tertiary: Domains as shown above http://www.people.virginia.edu/~rjh9u/gif/aminacid.gif Hydrophilic amino acids Fibrinogen: size and structure Size estimates Minor Axis 60 – 90 A Peptide chain in solution (R1, R2, R3, R4: Amino Acid Residues) http://bio.winona.msus.edu/berg/ChemStructures/Polypep2.gif Major Axis IR bands present in all protein backbones http://homepages.uc.edu/~retzings/fibrin2.htm (Hall CE, Slayter HS: The fibrinogen molecule: Its size, shape and mode of polymerization. J Biophys Biochem Cytol 5:11-15, 1959. Weisel JW, Stauffacher CV, Bullitt E, Cohen C: A model for fibrinogen: domains and sequence. Science 230:1388-1391, 1985.) AFM • Amide I band: C=O stretch • Amide II band: N-H deformation coupled to C-N stretch • Amide IV band: coupled C-N and C-O stretch 17 A • CH stretch 11 A 300 A • NH stretch 600 A CHICKEN FIBRINOGEN: Fibrinogen on mica Fibrinogen on graphite Marchin K. L. and Berrie C.L., Conformational changes in the plasma protein fibrinogen upon adsorption to graphite and mica investigated by atomic force microscopy, Langmuir 19 (2003) p.9883. Molecular Weight 54193 Number of Residues 491 R-CO-NH2 Amide II band Amide I band C-NH2 C=O Functional chemical group (olefins, esters, ethers, nitriles, thioethers, thioesters) acids or alcohols Germanium Tripod attachment Use hydrolysis of SiCl3-(CH2)16-COCl Determination of fibronectin structure from the Amide I spectrum -sheet -turn