* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download PDF
Extracellular matrix wikipedia , lookup
Tissue engineering wikipedia , lookup
Biochemical switches in the cell cycle wikipedia , lookup
Cytokinesis wikipedia , lookup
Cell encapsulation wikipedia , lookup
Cell growth wikipedia , lookup
Cell culture wikipedia , lookup
Cellular differentiation wikipedia , lookup
Organ-on-a-chip wikipedia , lookup
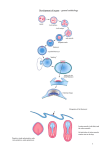
/. Embryol. exp. Morph. Vol. 53, pp. 269-289, 1979 Printed in Great Britain © Company of Biologists Limited 1979 269 Cell number in relation to primary pattern formation in the embryo of Xenopus laevis II. Sequential cell recruitment, and control of the cell cycle, during mesoderm formation By JONATHAN COOKE1 From the Division of Developmental Biology, National Institute for Medical Research, London SUMMARY Morphological evidence is presented that definitive mesoderm formation in Xenopus is best understood as extending to the end of the neurula phase of development. A process of recruitment of cells from the deep neurectoderm layers into mesodermal position and behaviour, strictly comparable with that already agreed to occur around the internal blastoporal 'lip' during gastrula stages, can be shown to continue at the posterior end of the presumptive body pattern up to stage 20 (earliest tail bud). Spatial patterns of incidence of mitosis are described for the fifteen hours of development between the late gastrula and stage 20-22. These are related to the onset of new cell behaviours and overt cyto-differentiations characterizing the dorsal axial pattern, which occur in cranio-caudal and then medio-lateral spatial sequence as development proceeds. A relatively abrupt cessation of mitosis, among hitherto asynchronously cycling cells, precedes the other changes at each level in the presumptive axial pattern. The widespread incidence of cells still in DNA synthesis, anterior to the last mitoses in the posterior-to-anterior developmental sequence of axial tissue, strongly suggests that cells of notochord and somites in their prolonged, non-cycling phase are G2-arrested, and thus tetraploid. This is discussed in relation to what is known of cell-cycle control in other situations. Best estimates for cell-cycle time in the still-dividing, posterior mesoderm of the neurula lie between 10 and 15 h. The supposition of continuing recruitment from neurectoderm can resolve an apparent discrepancy whereby total mesodermal cell number nevertheless contrives to double over a period of approximately 12 h during neurulation when most of the cells are leaving the cycle. Because of pre-existing evidence that cells maintain their relative positions (despite distortion) during the movements that form the mesodermal mantle, the patterns presented in this paper can be understood in two ways: as a temporal sequence of developmental events undergone by individual, posteriorly recruited cells as they achieve their final positions in the body pattern, or alternatively as a succession of wavefronts with respect to changes of cell state, passing obliquely across the presumptive body pattern in antero-posterior direction. These concepts are discussed briefly in relation to recent ideas about pattern formation in growing systems. 1 Author's address: Division of Developmental Biology, National Institute for Medical Research, Mill Hill, London, NW7 1AA, U.K. 18 EMB 53 270 J. COOKE INTRODUCTION Following the work of Nieuwkoop and his colleagues (Nieuwkoop & Florschutz, 1950; Sudarwati & Nieuwkoop, 1971) and of Lovtrup (Lovtrup, 1965), it has become clear that the classical picture of the cell movements constituting gastrulation and the formation of the amphibian body axis needs modification. That picture, dependent largely upon Vogt's (1929) interpretation of his vital staining experiments with Urodele (Newt) embryos, is still textbook lore despite the considerable body of more recent work (Keller, 1975, 1976; Nakatsuji, 1974, 1975) which confirms a reinterpretation of cell movements in the Anuran (Frog) gastrula. Although the classical picture probably needs modification for Urodeles, the geometrical differences in these fundamental, early cell movements, as between the major amphibian groups, could well justify a new assignment of class status to each of them within the vertebrates (Nieuwkoop & Satasurya, 1976). Keller (1975) points out that it is worth understanding the normal cellular movements in great detail for any organism which is to be used in attempts to understand, in a deeper sense, mechanisms underlying the early organization of embryos. The same might be said of what it is tempting to call the 'cellular demography' of the embryo; questions of the births and possible deaths of the cells composing various parts of the emerging body pattern, and of their recruitment from one cell layer into another as development proceeds. Study of these features, in turn, focuses attention on an aspect of early embryonic development which has been somewhat neglected hitherto. This is the onset of control of the cell cycle, via the intercellular communication systems that are organizing pattern formation, which contrasts with the earlier expression of regional schedules of mitotic rate inherited via the egg cytoplasm, that dominate the picture during cleavage. The present paper attempts to describe in outline the cell population dynamics, and spatio-temporal pattern of new cell behaviours, in the early Xenopus mesoderm during gastrulation, neurulation and into the earliest tail-bud stages. During this time, and primarily in this cell layer, the basic body plan becomes determined within a growing population of cells. It has proved practicable to use for this study the control embryos, sectioned as part of the study on the cell cycle in pattern regulation reported as Paper 1 of this series (Cooke, 1979). Although an exactly staged (Nieuwkoop & Faber, 1956) time-series of embryos, these had often been the progeny of different matings, developing on separate occasions at slightly different temperatures, and thus show apparent inconsistencies of absolute cell number and Mitotic Index in relation to developmental age. Their use in the study is possible because of confidence, through enough comparisons of true siblings fixed at separate time points, that within embryos the mesodermal cell cycle time as a whole stays constant or lengthens, and never decreases, across the period we are concerned with. One can therefore Cell number in Xenopus development. II 271 build up a picture of events by attending only to observed spatial patterns, within embryos, with respect to cell behaviour and cell-cycle parameters. Records of cell numbers, mitotic and labelling indices, and anatomical images of cell behaviour sampled at successive times in development have been pieced together to arrive at a description which, it is hoped, combines adequacy to its object with somewhat more brevity than those characterizing previous eras in embryology (e.g. Vogt, 1929). The (currently) somewhat unconventional style of the paper, whereby results distinctive to the present study are integrated with the findings of others throughout, is therefore considered unavoidable. The description confirms and extends Keller's (1976) finding of a late 'extension' of the classically defined period of gastrulation, after closure of the yolk plug (stage 12|-13, Nieuwkoop & Faber, 1956), in terms of the migration of cells away from a thick, internal circumblastoporal ring of mesoderm during subsequent stages. The description also resolves a prima facie inconsistency, referred to in the previous paper (Cooke, 1979), between the increase in total mesodermal cells over the period of development considered, the known length of the cell cycle, and the cessation of mitosis within much of the mesoderm within the same period. Findings that bear on the developmental control of the cell cycle during pattern formation are discussed in relation to a sparse literature on that subject (e.g. Maden, 1979; Tassava & Mescher, 1975). MATERIALS AND METHODS Embryos were from artificially induced spawning of Xenopus laevis, obtained at not less than 5-week intervals from each pair (see Cooke, 1979). Development had been without jelly or vitelline membrane from stages 9-10 until time of fixation, resting on glass in 1/10 strength amphibian saline. Laboratory temperature varied from 18 to 22 °C. Only embryos developing perfectly to external inspection were used, and were fixed, double-wax embedded and sectioned in exact transverse section at 7/tm for Feulgen +Light Green/Orange G staining as already described. Some 10% of embryos (3/32) showing abnormal intercellular and archenteric material, with some irregular large yolky cells (i.e. arrested cytokinesis) were discarded. Nuclei of the mesodermal cell layer, including the internal thickened circumblastoporal ring at earlier stages and posterior tail-bud mesoderm subsequently, as well as those of the deep marginal cells of the posterior neurectoderm (recruitment zone) in some cases, were scored in every fourth section throughout the embryos as interphase or mitotic (metaphase and anaphase; two visible anaphase sets of chormosomes scored as one mitosis). Eight synchronous sibling embryos were injected at precise stages, into the archenteron via the neural plate, with ca. 10% of their volume of a solution calculated to give 200 nCi tritiated thymidine (41 Ci/mmole, 1 /tCi//d) + l mM colchicine (Nakatsuji, personal communication) in 10 % Niu-Twitty saline in the 18-2 272 j . COOKE archenteron. Such an injection of [3H]thymidine is known to allow normal development, yet to label'S' phase mesodermal cells within 10 min, while such a colchicine injection arrests many cells reaching 'M' phase and yet allows substantial continuation of morphogenetic movements. The embryos were fixed after 1 h and sectioned in T.S. for staining as before, but in conjunction with dipping emulsion autoradiography (Ilford K2 emulsion; 14 days exposure; development in Kodak D19; Feulgen reaction before development and counterstaining after). The two most normally developing and perfectly sectioned examples of each of two ages were used to plot the incidence of cells in S phase, or having reached mitosis, within the last hour of development, in the progression of material from the posterior zone of mesoderm recruitment towards the anterior zone of differentiation. Certain embryos, having developed under similar conditions to those above, were fixed for 4 h in half-strength Karnovsky, rinsed overnight in 0-08 M phosphate buffer at pH 7-4, dehydrated and embedded in Araldite for 2 ^m sectioning, and stained with toluidine blue (Nakatsuji, 1974). The cell contacts and behaviour in the circumblastoporal and neighbouring zones was examined in this material. Description (a) Cell Movements As described by others (Nieuwkoop & Florschutz, 1950; Nakatsuji, 1974, 1975; Keller, 1975, 1976), the mesoderm is derived from the deeper cell layers of the marginal zone. These slide vegetally relative to the overlying surface cells, before rolling inwards around an 'internal lip' and moving again towards the animal pole to form the mesodermal mantle. Meanwhile, the surface neurectodermal layers of the marginal zone expand, and migrate upon the surface endoderm at their vegetal margin, finally to form the lateral and ventral blastoporal lips by a purse-string-like closure to engulf the endodermal mass. The only true surface invagination is of the future endodermal lining of the archenteron, dorsally, and at no time does mesoderm appear on the surface of the embryo. Fig. 1 depicts in a schematic way this conception of anuran gastrulation. Keller (1976) provides the most detailed fate map of the normal mesodermal cells so far justified by the data. His vital staining results suggest that in accord with classical views, and with evidence from the homologous movements in birds (Rosenquist & Dehaan, 1966), an early fate map is justified because mesodermal cells maintain their neighbourhood relationships, within the population as a whole, throughout the spatial transformations and distortions of the morphogenetic movements. This occurs despite a pronounced change in the character of mesodermal cells as they pass around the internal lip to leave the deep marginal zone with its compact, almost epithelial structure. They become individual cells, with more or less extensive processes and restricted contacts with one another and with the cell layers between which they are migrating. Cell number in Xenopus debelopment. II 273 n.ect end Fig. 1. Schematic representation of the cell movements of Xenopus ga.stru\a.t\on, in sagittal section. (A) During an early circular blastopore stage. (B) After closure of the blastopore. Definitive mesoderm, including the forming notochord on the middorsal (right) side of (B), is shown obliquely hatched, be = blastocoel or its remnants. arch — archenteron or its rudiment, n.ect = neurectoderm, two definitive cell layers thick at later stages and continuous throughout with the lining of the archenteron and hence with the yolky endoderm (= end), z.rec = deeper cell layers of the marginal neurectoderm; i.e. the zone of recruitment into mesoderm shown by arrows. Fig. 2 A-D, from Araldite sections in the present work, shows cells at the neurectoderm-mesoderm transition point, seen at progressively later times in gastrulation; that is, at the origin of progressively more posterior mesoderm. It can be seen that the appearance in the region of the blastopore lip, which correlates at recognized gastrula stages with a well substantiated transformation of cells' behaviour as they are recruited from one layer into another, is evident until stage 19. It exists in a substantial zone around the closed blastopore at midneurula stages (see Fig. 2(C), and also recorded at stage 15). Nakatsuji (1974) and Keller & Schoenwolf (1977) have described these predifferentiated mesodermal cells (at least for anterior regions of the presumptive body pattern) at the plastic section and scanning E.M. levels, while Kubota & Durston (1978) suggest from a time-lapse cinemicrographic study of opened gastrulae, how they might maintain their relative positions while migrating in this way. In regions only just anterior to the zone of recruitment, mesoderm of stages from 12 on through neurulation exists as a thick internal collar, now discrete from the outer neurectoderm (which is clearly two cell layers thick, especially at levels well anterior to the recruitment zone). In still more anterior sections, up to the anterior mesodermal border, only one or two layers of mesodermal cells 274 J. COOKE ng bp Fig. 2. For legend see opposite. Cell number in Xenopus development. II 275 separate ectoderm and endoderm until, beginning anteriorly at stage 12 J/13 and spreading laterally and posteriorly, cells progressively aggregate dorsalwards preparatory to the individuation of axial structures (Mookerjee, Deuchar & Waddington, 1953; Hamilton, 1969; Nieuwkoop & Faber, 1956). It is important to realize though that long after this, the layer of very flattened (because stretched) cells of the lateral and ventral mesoderm is still continuous with the dorsal axial aggregate, making the mesoderm still a coherent cylindrical mantle of tissue. From stage 19 on, with the onset of tail-bud development, the mesodermal cell population for the latter appears to be derived by a shift of the population of the circumblastoporal collar into the definitive location, posterior and dorsal to the blastopore, in which development of the axis continues. Such later development is obviously homologous, when it occurs, with the cellular events and movements of the earlier more anterior axial morphogenesis, and continues smoothly on from the latter in its timing. The initial formation of the tail-bud coincides with an apparent cessation of cell recruitment into the mesoderm as a whole, from the outer cell layers (seeFig. 2D-F). At stage 20, though no longer by stage 22, areas near the posterior ecto-endodermal boundary are still seen where the adhesive properties which keep definitive mesoderm segregated from the former, epithelioid cell layers seem ill-developed (Fig. 3A, B). It is important to realize that this recruitment, before stage 20, is itself distinct from the process whereby cells are 'paid out' from the population of the thick circumblastoporal collar, already definitive mesoderm, into more anterior and dorsal regions as development proceeds. Keller (1976) has already described the latter process. Because of the obviously continuous mesodermal recruitment from 'official' gastrula stages through to stage 20 (Fig. 2A-D), it is meaningful to say that gastrulation continues through neural induction and during the formation of FIGURE 2 Camera lucida tracing of cell outlines in 2/*m epon sections through the zone of mesodermal recruitment and tail bud (A) Transverse section, some 50/^m anterior to the zone of recruitment, at stage 13. (B) Oblique section through the zone of recruitment, as shown in inset; stage 12£ gastrula. (C) Nearly transverse section through zone of mesodermal recruitment around blastopore; stage-14 neurula. Ventral part of section progresses anteriorly out of recruitment zone. (D) Transverse section through small, very posterior zone of recruitment; stage-19 neurula blastopore region. (E) Transverse section through level of ventrally rotated blastopore showing no appearance of recruitment; stage-22 tail bud. (F) Transverse section through tail-bud tip, stage 22, showing discrete mesoderm without appearance of recruitment. z.rec = zone of mesoderm recruitment; — = ecto-mesodermal boundary; bp = blastopore or archenteron cavity; ng = neural groove; mes = mesoderm. 276 J. COOKE Fig. 3. 2/im transverse epon sections through stage 20; toluidine blue staining. (A) Level of blastopore, now moving ventrally. (B) Region of tail-bud mesoderm, posterior to blastopore. Cells of classical germ layers, ect = neurectodrem, mes = mesoderm, end = endoderm. Heavy arrows point out regions near ecto-endodermal junction where mesodermal recruitment has been occurring until this time in development, and where, in cell-behavioural terms, the boundaries between these definitive cell layers and mesoderm is still less strict than is characteristic elsewhere. the first few somites (Nieuwkoop & Faber, 1956; Hamilton, 1969). This should not surprise us in view of the obvious continuation of node regression and the accompanying invagination of mesoderm posteriorly, while the anterior axis is already highly differentiated, in development of the telolecithal eggs of birds and fish (Hamburger & Hamilton, 1951; Oppenheimer, 1947). What is observed here is quite equivalent, in the 'internalized' gastrulation ofXenopus, to the posterior extension of the fate map of the mesoderm onto the exterior of the early Urodele neurula (Nakamura, 1942). Individuation of the notochord is first marked by behaviour among a middorsal strip of cells which segregate from their neighbours to form a rod, beginning anteriorly at stage 12£ and extending rapidly to approach the circumblastoporal collar by stage 13 (see Mookerjee et al. 1953). From stage 13£ on, the distinctive notochordal behaviour is evident in mid-dorsal cells even Cell number in Xenopus development. II 277 Notochord Gut end Fig. 4. Camera lucida outlines of cell layers to show structure at the posterior origin of notochord; stage-14 neurula. (A-D) Four transverse sectional appearances, from epon sections 8-15 fim apart in posterior-anterior sequence, showing the continuity of the rod of notochordal cells with the mid dorsal area of the zone of mesodermal recruitment (cross-hatched), which is itself derived from the deep layers beneath the topologically continuous epithelial sheet of the neurectoderm (root of the neural plate) and endoderm (root of the yolk plug), shown oblique hatched. Definitive nonnotochordal mesoderm is shown stippled. Labelling as before. within the posterior zone of recruitment. Fig. 4 shows transverse sections in postero-anterior order, and thus representing a time sequence in development, depicting the constitutive cell layers of the embryo in this region. From the cell contact relationships as seen in epon sections it appeared likely that cells are recruited directly into notochordal behaviour from the deep neurectoderm, now the root of the neural plate, at its junction with the endoderm of the archenteron roof. (Note that this represents a point beneath the original superficial marginal zone, now folded on itself and expanded as part of gastrulation. See Fig. IB, and Keller, 1975.) It also remained possible however that a defined notochordal cell population achieved by, say, stage 13£, creates a 278 J. C O O K E cranio-caudally elongating structure from then on while the other, more lateral mesoderm continues to be recruited as described earlier. Since by stages 131-14, mitosis is a rare occurrence even at the posterior limit of notochord, the situation was easily assessed by making equivalent estimates of total cell numbers in the notochords of exactly synchronous sibling embryos, fixed at stage 14| and 5 | h later (22 °C) at stage 19. The mesoderm cell number as a whole increases by a factor of some 35 % over this period, because of posterior recruitment and then the maintenance of a certain probability of mitosis among many of the cells (see Cooke, 1979, and next section). The notochord cell number, meanwhile, increases by a factor whose best estimate is some 22 %, and it has at most a fraction of the general mesodermal incidence of mitosis, restricted to its caudal origin. Thus the four stage-14| embryos had notochords consisting of an estimated 825, 705, 900 and 835 cells. Their four stage-19 siblings had 1010, 990, 935 and 1005 cells. One mitotic figure each was seen, in the putative zone of recruitment, in 3 of the 8 embryos. Material sectioned in the horizontal and sagittal planes at these stages shows that shape changes in notochord cells are not yet significant enough to bias the number estimate from nuclei in T.S., over the stated period. (See Cooke, 1979, and Abercrombie, 1946, for details of cell number estimation in this work.) The localized appearance of cell-behaviour change at the notochordal root beneath the neurectodermal-endodermal boundary is also confirmed. Advanced tail-bud larvae definitely have a greatly enhanced notochord cell population, so that on balance it seems highly probable that the notochord rudiment is being added to by recruitment as is the rest of the mesoderm during neurula stages, even though mitosis among the cells so recruited stops immediately rather than after a few hours as for presumptive somites. (b) Mitosis within the mesoderm during axis formation It seems safe to conclude that cell death plays no normal role in Xenopus development over the stages being considered here. At no time in pre-larval stages, investigated with any nuclear staining technique in this laboratory, has pyknosis been seen other than in cells fixed just after obvious wounding. Otherwise only healthy interphase or mitotic nuclear images are seen (including a proportion of multinucleate cells in endodermal regions). The total cell number in the definitive mesoderm as development proceeds, therefore, is a function of recruitment from the deep marginal layers (up to stage 19) plus the incidence of mitosis among the cells thus recruited, with only the latter process contributing from tail-bud stages (20) on. Fig. 5 presents data on the patterns of incidence of mitosis with respect to position in the body axis, sampled between stages 12| and 22, pooled from two embryos at each stage sampled in transverse section. Figs. 6A and B are deduced representations of the relative densities of mitoses in embryonic mesoderm, at stage 12-|- and from stage 14 onward, respectively. How is the cell population built up around the time of axis formation? The Cell number in Xenopus development. II 279 A 12 Fig. 5. Distribution of mitoses in the developing mesoderm at various stages. Mitotic index % is shown on the ordinates, and each sample point on the abscissae represents the mean mesodermal index scored cumulatively on four successive transverse 7 jum sections, 28 //m apart, in each of two synchronous embryos sectioned in parallel from posterior (left) to anterior (right) ends. Each value thus derives from counts of some 2-500 cells, with the solid plots representing the presumptive dorsal tissues (notochord and somites) and the dashed plots the presumptive lateral and ventral mesoderm. The prechordal plate area tends to appear in both plots, becoming parallel to the plane of sectioning at the anterior region afters tage 12&. Bold arrows in D.E.F. mark posterior limit of somite rotation. (A) Stage 12S. (B) Stage 13. (C) Stage 14. (D) Stage 19. (E) Stage 20. (F) Stage 22. overall picture from these data is of a homogeneous rate, in mesoderm and in the deep marginal layers which are giving rise to it by recruitment, at stages prior to 12 (small yolk plug). From stage 12| a wavefront marked by a sharp decrease in the probability of mitosis, passes antero-posteriorly through the notochordal and then the axial mesodermal regions. Thus by stage 13, about 1 h later, mitosis has virtually ceased in all but the posterior third of the presumptive notochord and is greatly reduced in most of the presumptive somite material, while by stage 14 (another hour later) a stable situation has been reached which is maintained 280 J. COOKE Dorsal Ventral Fig. 6. Representation of mesodermal mantles to show the spatial pattern of incidence of mitoses. (A) Stage 12£. (B) Stages 14 onwards. In each diagram, density of stippling is related to the absolute incidence of mitoses (Mitotic Index), pc, Prechordal area; dm, dorsal midline; be,rim of blastocoele remnant; 6/?,internal blastopore; cbpc,ckcumblastoporal collar (thick mesoderm). Mesoderms are represented from a lateral, but slightly posterior and slightly dorsal, aspect. until the close of mesodermal recruitment, stage 19/20, and in modified form thereafter. During this period, there is still a homogeneous mitotic rate among the deep cells whose posterior sliding beneath the epidermis, then delamination and change of behaviour in the circumblastoporal region, is giving rise to further mesoderm. This mitotic rate applies also in the collar of newly formed mesoderm itself except in the dorsal midline where behaviour is notochordal even in the most posterior cells, and mitosis accordingly absent. But mitotic index in the dorsal half of the primitive mantle, the presumptive somite columns, falls off very sharply in an anterior direction to the almost zero level (0-1 %) that characterizes this tissue during the subsequent episode of somite morphogenesis that progresses from the anterior end. At each stage, however, mitotic index in the lateral plate and anterolateral (heart, pro-nephric, etc.) mesoderm remains homogeneous with that of the recruitment zone and posterior mesodermal collar. The precocious cessation of mitosis among cells recruited into the notochord is not unexpected in view of the relatively earlier onset of cell behaviour marking the morphogenesis of this mid-dorsal part of the pattern. Cell number in Xenopus development. II 281 From stages 20 onwards through the laying down of the axis, the situation differs only in that, since no further cells are recruited from outside definitive mesoderm, further increase in numbers of (temporarily) post-mitotic cells to form somites and notochord occurs solely by mitosis in a small posterior terminal region of tissue. This region seems to be the product of dorsal/posterior migration e« masse from the previous circumblastoporal collar or reservoir of cells. A region remaining outside this pattern, and not mentioned hitherto, lies at the antero-dorsal and lateral edges of the mesodermal mantle. It is the prechordal plate or precursor of primary head mesenchyme. From mid-gastrulation (11 ?,) through to stage 20, after which its cells disperse to give rise to the loosely arranged mesenchyme, its mitotic index is significantly enhanced over that of other still-dividing mesodermal regions. Since mesodermal tissue is being added by recruitment posteriorly, the relative extent in the embryo, of this more rapidly dividing region, tends to decrease as development proceeds. This is particularly so when stage \2\ is compared with subsequent stages (see Fig. 5 and 6), because of the incompletely anterior and vertical position of the prechordal area in the unfinished gastrulation movements of the former stage. But there is no evidence that the area of high mitosis, in terms of the pattern of the future body, diminishes before dispersal of the pre-chordal plate. Onset of heightened mitotic index in anterior sections always follows the anterior limit of somites and notochord. In stages 20 and 22 there was evidence for greatly enhanced mitosis among groups of cells at the somite/lateral plate boundary, at the expected anteroposterior level of the pro-nephros (see Fig. 5E, F). But due to the small numbers of these nuclei, more than the four embryos involved would need to be scanned for confidence in the reality of the finding. The general mesodermal mitotic index (i.e. excluding the dorsal axis of near zero M.I. and the pre-chordal area of enhanced M.I.) varies significantly, between egg batches observed in this work. Ambient temperature differences, and genetic variance of the adult female toads, most probably account for such variation in the absolute proportion of the cell cycle occupied by mitosis, at early morphogenetic stages (Dettlaff, 1964; Chulitskaia, 1970). Enough comparisons nevertheless exist between sibling embryos developing under the same conditions on the same occasions, but fixed at different stages, to show that there is a slow decrease in mitotic index in the recruitment zone and still-dividing mesoderm as development proceeds over the period studied. This is typically some 6% in mid-gastrulae, half this value by neural plate stages, and decreases more slowly with time thereafter to 1-5% typically at stage 20 (see Cooke, 1979). It is notoriously difficult to establish the mean cycle time in a growing cell population in vivo, other than by direct, successive counts. The dual problems of recruitment, and of removal from the cycling pool prior to differentiation in the dorsal regions of the axis, render this impossible in the present case. In midneurulae labelled by injection with [3H]thymidine for \, \\ and 2\ h before 282 J. C O O K E fixation (two per time point per experiment) in this laboratory, labelling indices in lateral plate and circumblastoporal mesoderm were 54-2%, 63-0% and 69-2% respectively in one experiment, and 51-0%, 60-9%, 68-7% respectively in the other. No metaphase figures were labelled even after 2 | h. Since none of the increase in labelling index between | and 2 | h could thus have been due to successive incorporation in two S phases, the best estimate is that some 7-5 % or some 9 % of cells were entering S phase per hour in the two experiments, giving a cycle time of some 13^ h or 11 h respectively. This estimate tallies fairly well with the indirect one derived from comparison with the published values for M.I. and independently estimated cell cycle time for Xenopus endoderm (Graham & Morgan, 1966), on the assumption that differences of cell cycle time within early embryos of a species, if present, largely reflect differences in phases other than mitosis (M). See also Deuchar (1958) and Cooke (1973). (c) Observations relevant to cell cycle control Fig. 7 shows results from an experiment in which exactly synchronous sibling embryos developing at a constant 22 °C were injected with [3H]thymidine and colchicine into the archenteron, 1 h before fixation at stage 14| or (5J h older) stage 19. Thus mesodermal cells entering S phase and many of those entering M, within the labelling period, were recorded as grain-covered nuclei or mitotic arrests, respectively. Perfect embryos of each stage were sectioned in exact T.S., and the incidence of S phase and of mitotic arrest plotted in cells leaving the zone of recruitment and passing anteriorly to join the differentiating dorsal mesodermal axis. Posteriorly, the expected slightly enhanced incidence of mitotic figures (ca. 6%), some of them abnormal, is seen in presumptive dorsal axial cells. The expected incidence of nuclei covered by many silver grains in contrast to their unlabelled neighbours is also seen. No mitotic figures are labelled, and among notochord cells there are interphase nuclei labelled at typical intensity, as well as unlabelled ones. At stage 14£ there is a clear pattern, seen in Fig. 7, whereby the mitotic index drops rapidly to zero as one progresses anteriorly along the dorsal axis, before the zone where dorsal mesodermal convergence is occurring (cf. Fig. 5B, C, D), but leaves a normal incidence of labelled interphase nuclei throughout this zone. The latter incidence only decreases gradually towards zero, accompanied by diminished mean labelling intensity, near the anterior end of the somite columns. Labelling also decreases in incidence and intensity in anterior progression through the notochord, but reaches zero sooner than in the presumptive paraxial tissue. At stage 19 the picture is obscured in that the greater (anterior) portion of the axis contains neither mitotic figures nor label, even though both mitotic arrests and densely labelled nuclei are abundant in the overlying neural tube, proving easy access to cells of colcemid and thymidine from the archenteron. But again, Cell number in Xenopus development. II 283 100, 20 Fig. 7. Incidence of cells, in S phase and arriving at mitosis, during the last hour of development before stage 14$, in postero-anterior progression from the zone of recruitment in dorsal axial mesoderm. Total dorsal mesodermal cells sampled (clear columns), cells in S phase (stippled) and cells having arrived at mitosis (solid) are recorded as pooled from two stage-14$ embryos, in every fourth transverse section in series passing anteriorly from the zone of mesodermal recruitment. For further explanation see text. The two embryos had an estimated 825 and 855 notochordal cells, including posteriorly some 210 and 52 S phase cells, and no mitoses. Bold arrow represents the approx. level where dorsal convergence of somitic mesoderm commences. in anterior progression through a very short zone of newly recruited dorsal mesoderm, mitoses disappear before labelling. Because of posterior recruitment, the progression from posterior to anterior in the mesoderm can be said to encounter cells at progressively more advanced points along a developmental sequence. The observed spatial patterns of cells passing through mitosis and through S phase during 1 h, i.e. a brief period in relation to this whole developmental sequence, were highly constant in each of the four embryos of the two developmental stages. They varied only in the precise axial positions at which the S phase incidence decreased to zero, as if this feature itself were the most sensitive index of developmental age. The data are evidence that the prolonged arrest of the cell cycle, just preceding the individuation of somites and notochord in Xenopus embryos, operates to collect the asynchronously cycling cells in G2 by prohibiting mitosis, rather than by retaining cells in a phase equivalent to Gx by prohibiting the onset of DNA replication. D I S C U S S I O N : AN I N T E G R A T E D D E S C R I P T I O N During the movements that give rise to the mesodermal mantle in amphibian embryos, there is little shifting of the relative neighbourhood relationships between individual mesodermal cells despite the extreme deformations undergone by the cell-sheet as a whole. In this respect, although mesoderm cells have a relatively 'independent' appearance, the whole population acts less like a liquid 284 J. C O O K E than like a highly deformable solid (as in a rubber sheet). To the extent that this is so the relationship, between cells' positions within mesoderm during gastrulation and the positions they or their descendents finally occupy in the axial pattern of differentiations, constitutes what is known as a conformal mapping. The vital staining experiments of Keller (1976) and the cinemicrographic observations of Kubota & Durston (1978) on mesoderm migration in situ, offer two sorts of evidence that gastrulation has this character. The grafting of patches of labelled cells into the chick blastoderm (Rosenquist & DeHaan, 1966), and the finding of localized, coherent patches of disrupted somite tissue after short heat shocks given to Xenopus even in the early gastrula stage (Cooke, 1978), provide further evidence for prolonged maintenance of neighbourhood relationships in mesodermal development. This knowledge allows us to conceptualize in an integrated way the events, hitherto described piecemeal in terms of various cell activities, in this paper. They will be considered first as a constant developmental sequence, marked by relatively sudden changes of cell state, undergone in serial order by the cells according to presumptive position along the body pattern. Alternatively they can be viewed as a series of oblique wavefronts of visible, relatively rapid cell change, sweeping through the body pattern in a head-to-tail direction with the dorsal midline in the lead. This second way of viewing the events poses the question of the sort of primary, hidden organization that could give rise to the final, visible pattern achieved. The changes accompanying initial recruitment of cells into mesoderm, in the anuran gastrula, now seem very similar to those playing the equivalent part in the development of telolecithal embryos such as birds and fish. The molecular bases of such co-ordinated changes are not understood, though studies of the new properties of individual cells at gastrulation have been made at other levels by Holftreter (1943) and Johnson (1970). The next milestone in each dorsal mesodermal cell's development seems to be a relatively sudden prohibition of mitosis. The cell cycle seems to be occurring with random phase in the tissue before a sharp drop in the M.I., effectively to zero, sweeps through it over a time equivalent to less than one normal cycle. This zone or wavefront of mitotic cessation is definitely posterior, at any given time, to the much more gradual one across which the incidence of S-phase cells then declines from normal values to zero among progressively 'older', more anterior cells. Mitosis is therefore the first visible cell cycle phase to which entry is prohibited in mesoderm cells, following posterior recruitment from deep neurectoderm. This particular cycle-block therefore appears to operate on tetraploid cells between S phase and mitosis, collecting them up into a G2 condition. By themselves however, these observations tell us nothing of the nature of the blocking signal, or of when this is received by each cell, except that passage of the signal is too rapid and coherent to depend on the cycle itself, as the latter is long and randomly phased among cells. The signal must gate the cycle, rather than itself being a product of the cycle. Cell number in Xenopus development. II 285 Most postmitotic (and thus mortal) cells in the organization of adult tissues are thought to be in a pre-replicative condition equivalent to Gx (Go), as are cells reversibly inhibited from dividing by limitation of various 'factors' in tissue culture media. Little attention has so far been paid to possible modes of cell cycle control during early development or pattern regeneration, which may be an integral part of morphogenesis, as opposed to the humoral controls that maintain homeostatis during later life. Thus it is not known if G2 stasis, or rate control during phases other than the traditional GX/GQ switch, is a regular feature in early development. In the present case cells temporarily cease divisions during a prolonged phase of morphogenesis and differentiation. A further study is planned, by using scanning densitometry, to estimate DNA content of cells at different positions in the dorsal axis, and thus check the inference of G2 arrest derived from the present evidence. It may also be possible to reveal whether the gradual anterior decline in labelling intensity (in addition to that in incidence) is due to a normally low rate of incorporation in late S phase, or whether the rate of DNA synthesis itself is subject to great slowing as part of the control process. Cells of the regenerating amphibian limb blastema, arrested because of denervation of the limb, are reported to include G2 cells (Tassava & Mescher, 1975), though there is evidence that conflicts with this (Maden, 1979). The next change of state, ensuing a while after each cell becomes postmitotic, is its participation in the defined series of alterations in contact behaviour, adhesivity and shape that marks the individuation of notochord and somites at each successive axial level. The process of notochord formation, initiated by the wavefront of new behaviour passing down the median dorsal region, has been described in an amphibian by Mookerjee et at. (1953), while the detailed cell movements of Xenopus somite morphogenesis have been described by Hamilton (1969). Bellairs (1979) is currently making a detailed study of the parameters of cellular change that might explain the observed behaviour in somite morphogenesis. The cytodifferentiations finally found among the dorsal axial cells, notably those of notochord and somite muscle, are also achieved in a time-graded, wavelike manner corresponding to the previous orderings of recruitment, mitotic blockade, somite segmentation, etc. It is not known at what point in this sequence molecular differentiation, or determination for the various final cell types, begins. There is some evidence, in relation to distinctive muscle proteins, that synthesis begins early in gastrulation, If so, it pre-dates what embryologists have always considered to be the period of determination for somite development among particular cells, as well as somite morphogenesis. Nevertheless the final, cytologically visible stages of notochord and somite development do occur in such a spatial sequence as to constitute a final wavefront in the series. Thus over a long period, from histodifferentiation in the anterior somites around the latest stages mentioned in this paper, onwards into larval life, any stage of 19 EMB 53 286 J. COOKE development of the dorsal axis may be found at some point in a complete series of transverse sections. Stage 20 marks a time when the sequence of wavefronts changes in one respect. From this time on, instead of recruitment from the deep neurectoderm being the first event in the history of each mesoderm cell or its immediate mother cell, all new cells supplied to the developing axis can come from the posterior zone of dividing but already recruited mesoderm. Cell production in the latter keeps pace with the uniform backward progress of the other wavefronts after mid-20's stages, giving rise to a roughly constant sized 'pre'-differentiated zone. Because of this change in the situation, cells destined for successively more caudal somites, far down the axis, are the progeny of increasing time or number of rounds of the cell cycle spent in the terminal mesoderm. In the case of about the first twelve somites, on the other hand, it seems unlikely that all the constituent cells will have divided even once since their recruitment into mesoderm (though some of them certainly do so). Cell position in this region of the somite sequence must correlate very strongly with the temporal sequence of mesodermal recruitment, and may coincide with it completely. Description of these mesodermal events, including initial recruitment from deep marginal neurectoderm, as a succession of wavefronts passing through the future body pattern, raises an important question about the underlying organization. Such wavefronts could be the final expressions of a programmed gradation in rates of passage through a developmental sequence by mesodermal cells, with the programming occurring in response to initial position within a compressed pattern-forming field in the marginal zone at the onset of gastrulation, and the highly organized wavefronts at later stages due to the maintenance of neighbour relations among cells during mesoderm recruitment. Programmed timing during development could then be thought of as an aspect of response to the primary dynamic of a regulative field, whether the latter were a hidden wave or some other positional signal (Zeeman, 1974, 1976; Wolpert, 1971; Goodwin & Cohen, 1969). Alternatively, the observed wavefronts could be more direct responses to propagated changes in conditions affecting mesodermal cell behaviour, such changes (e.g. in packing density, intercellular substances, etc.) always beginning at the presumptive anterior end of the pattern'because the firstrecruited, thus 'oldest' cells are there. At present, there is considerable evidence in favour of very early programming within mesodermal tissue with respect to local timing of movements (Cooke, 1972, 1975a; Bautzmann, 1933; Deuchar & Burgess, 1967), and with respect to specific contributions to the future pattern of differentiation (Kieny, Mauger & Sengel, 1972; Waddington, 1941). Thus visible axis formation may have more the character of a 'Kinematic' response (in Zeeman's terminology) to quite early pre-programming. Finally, it is interesting to note that the slight change of organization at around stage 20, marked by the cessation of true recruitment into mesoderm and its replacement by the terminal dividing zone, renders the development of Cell number in Xenopus development. II 287 the later parts of the axis more closely similar to that which has been ascribed to the avian limb bud. This is because cells of new positional value are generated only after mitoses undergone within a very narrow zone at one end of the system, positional characteristics (including timing) being very stable elsewhere among the developmentally further advanced cells. Experimental evidence for such a statement (regardless of the detailed mechanism) has been fully described for the chick limb bud (Summerbell, Lewis &Wolpert, 1973; Summerbell& Lewis, 1975) and the similarity of organization for the posterior somite pattern of Xenopus noted (Cooke, 19756, 1977). The only obviously different behaviour between the two systems is that the limb continues true growth (in a locally pre-programmed way) throughout its pattern, even though cells which extend the pattern are only produced at one end. Within the amphibian posterior axis, the growth at the locality where new pattern values can be produced is at that time the only true growth taking place in the system. The fundamental changeover from operation of a regulative system throughout the presumptive mesoderm (i.e. before gastrulation, Cooke, 19756), to a condition where only developmental time and growth are involved in production of new pattern, comes much earlier than the onset of tail-bud development. It seems to have occurred before the earliest neurula stage. But for some while, the process of posterior recruitment of cells into definitive mesoderm obscures the fact that the pattern is now forming by the spread of wavefronts of differentiation towards a zone where new cells are continually being produced to accommodate them. This work is supported by the Medical Research Council of Great Britain. REFERENCES M. (1946). Estimation of nuclear population from microtome sections. Anat. Rec. 94, 239-247. BAUTZMANN, H. (1933). t)ber determinationsgrad und Wirkungsbehiehungen der Randzonenteilanlagen. Wihelm Roux Arch. EntwMech. Org. 128, 665-710. BELLAIRS, R. (1979). The mechanism of somite segmentation in the chick embryo. /. Embryol. exp. Morph. 51, 227-243. CHULITSKAIA, E. V. (1970). Desynchronization of cell divisions in the course of egg cleavage and an attempt at experimental shift of its onset. /. Embryol. exp. Morph. 23, 359-374. COOKE, J. (1972). Properties of the primary organisation field in the embryo of Xenopus laevis. III. Retention of polarity in cell groups excised from the region of the early organiser. /. Embryol. exp. Morph. 28, 47-56. COOKE, J. (1973). Properties of the primary organisation field in the embryo of Xenopus laevis. IV. Pattern formation and regulation following early inhibition of mitosis. /. Embryol. exp. Morph. 30, 49-62. COOKE, J. (1975 a). Local autonomy of gastrulation movements after dorsal lip removal in two anuran amphibians. /. Embryol. exp. Morph. 33, 147-157. COOKE, J. (19756). Control of somite number during development of a vertebrate, Xenopus laevis. Nature, Lond. 254, 196-199. COOKE, J. (1977). The control of somite number. In Vertebrate Limb and Somite Morphogenesis, (ed. Ede, Hinchliffe & Balls). Symp. Soc. devl Biol. 3, pp. 433-448. ABERCROMBIE, 19-2 288 J. COOKE J. (1978). Somite abnormalities caused by short heat shocks to pre-neurula stages of Xenopus laevis. J. Embryol. exp. Morph. 45, 283-294. COOKE, J. (1979). Cell number in relation to primary pattern formation in the embryo of Xenopus laevis. I. The cell cycle during new pattern formation in response to implanted organisers. /. Embryol. exp. Morph. 51, 165-182. DETTLAFF, T. A. (1964). Cell divisions, duration of interkinetic states and differentiation in early stages of embryonic development. In Adv. Morphogen. 3, 323-362. DEUCHAR, E. M. (1958). Regional differences in catheptic activity in Xenopus laevis embryos. /. Embryol. exp. Morph. 6, 223-237. DEUCHAR, E. M. & BURGESS, A. M. C. (1967). Somite segmentation in amphibian embryos: is there a transmitted mechanism? /. Embryol. exp. Morph. 17, 349-359. GOODWIN, B. C. & COHEN, M. H. (1969). A phase shift model for the spatial and temporal organisation of developing systems. / . theoret. Biol. 25, 49-107. GRAHAM, C. F. & MORGAN, R. W. (1966). Changes in the cell cycle during early amphibian development. Devi Biol. 14, 439-460. HAMBURGER, V. & HAMILTON, H. L. (1951). A series of normal stages in the development of the chick embryo. /. Morph. 88, 49-92. HAMILTON, L. (1969). The formation of somites in Xenopus laevis. J. Embryol. exp. Morph. 22, 253-267. HOLTFRETER, J. (1943). A study of the mechanics of gastrulation. /. exp. Zool. 94, 261-318. JOHNSON, K. E. (1970). The role of changes in cell contact behaviour in amphibian gastrulation. /. exp. Zool. 175 (4), 391-428. KELLER, R. E. (1975). Dye mapping of the gastrula and neurula of Xenopus laevis. I. Prospective areas and morphogenetic movements of the superficial layer. Devi Biol. 42,222-241. KELLER, R. E. (1976). Dye mapping of the gastrula and neurula of Xenopus laevis. II. Prospective areas and morphogenetic movements of the deep layer. Devi Biol. 51, 118-137 KELLER, R. E. & SCHOENWOLF, G. C. (1977). An S.E.M. study of cellular morphology, contact and arrangement, as related to gastrulation in Xenopus laevis. Wilhelm Roux' Arch. Devi Biol. 182, 165-186. KIENY, M., MAUGER, A. & SENGEL, P. (1972). Early regionalization of the somitic mesoderm as studied by the development of the axial skeleton of the chick embryo. Devi Biol. 28, 142-161. KUBOTA, H. Y. & DURSTON, A. J. (1978). Cinematographical study of cell migration in the opened gastrula of Ambystoma mexicanum. J. Embryol exp. Morph. 44, 71-80. LOVTRUP, S. (1965). Morphogenesis in the amphibian embryo: gastrulation and neurulation. COOKE, Zoologica gothoburgensia 1, 1-139. MADEN, M. (1979). Neurotrophic and X-ray blocks in the blastemal cell-cycle. /. Embryol. exp. Morph. 48, 169-175. MOOKERJEE, S., DEUCHAR, E. M. & WADDINGTON, C. H. (1953). The morphogenesis of the notochord in Amphibia. /. Embryol. exp. Morph. 1, 399-412. NAKAMURA, O. (1942). Die Entwicklung der hinteren Korperhalfte bei Urodelen. Annotnes zool. jap. 21, 167-185. NAKATSUJI, N. (1974). Studies on the gastrulation of amphibian embryos: Pseudopodia in the gastrula of Bufo japonicus and their significance to gastrulation. /. Embryol. exp. Morph. 32, 785-804. NAKATSUJI, N. (1975). Studies on the gastrulation of amphibian embryos: Cell movement during gastrulation in Xenopus laevis embryos. Wilhelm Roux. Arch. EntwMech. Org. 178, 1-14. NIEUWKOOP, P. D. & FABER, J. (1956). Normal table of Xenopus laevis (Daudin), 2nd ed. Amsterdam: North Holland. NIEUWKOOP, P. D. & FLORSCHUTZ, P. A. (1950). Quelques characteres speciaux de la gas- trulation et de la neurulation de l'ceuf de Xenopus laevis (Daudin) et de quelques autres Anoures. I. Etude descriptive. Archs Biol. (Liege) 61, 113-150. NIEUWKOOP, P. D. & SATASURYA, L. A. (1976). Embryological evidence for a possible polyphyletic origin of recent amphibians. /. Embryol. exp. Morph. 35, 159-167. OPPENHEIMER, J. M. (1947). Organisation of the teleost blastoderm. Q. Rev. Biol. 22, 105. Cell number in Xenopus development. II 289 ROSENQUIST, G. C. & DEHAAN, R. L. (1966). Migration of pre-cardiac cells in the chick embryo; a radioautoradiographic study. Publs. Carnegie Instn Wash. 625, Contributions to Embryology (38), 111-121. SUDARWATI, S. & NIEUWKOOP, P. D. (1971). Mesoderm formation in the Anuran, Xenopus laevis (Daudin). Wilhelm Roux. Arch. EntwMech. Org. 166, 189-204. SUMMERBELL, D., LEWIS, J. H. & WOLPERT, L. (1973). Positional information in chick limb morphogenesis. Nature, Lond. 244, 492-496. SUMMERBELL, D. & LEWIS, J. H. (1975). Time, place and positional value in the chick limb bud. J. Embryol. exp. Morph. 33 (3), 621-643. TASSAVA, R. A. & MESCHER, G. L. (1975). The roles of injury, nerves and the wound epidermis during the initiation of amphibian limb regeneration. Differentiation 4, 23-24. VOGT, W. (1929). Gestaltungsanalyse am Amphibienkeim mit ortlicher. Vitalfarbung. II. Gastrulation und Mesodermbildung bei Urodelen und Anuren. Wilhelm Roux. Arch. EntwMech. Org. 120, 385-706. WADDINGTON, C. H. (1941). Translocations of the organiser in the gastrula of Discoglossus, Proc. Zool. Soc. Lond. A 111, 189-198. WOLPERT, L. (1971). Positional information and pattern formation. Curr. Top. devl Biol. 6, 183-223. ZEEMAN, E. C. (1974). Primary and secondary waves in developmental biology. In Lectures on Mathematics in the Life Sciences, 7, pp. 69-161. Amer. Math. Soc. (Rhode Island). ZEEMAN, E. C. (1976). Gastrulation and formation of somites in amphibia and birds. In Structural Stability: the Theory of Catastrophes and Applications in the Life Sciences. Springer Lecture Notes in Mathematics, no. 525, pp. 396-401. (Received 8 February 1979, revised / May 1979)