* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Chapter 7 Notes - MDC Faculty Home Pages
Nicotinamide adenine dinucleotide wikipedia , lookup
NADH:ubiquinone oxidoreductase (H+-translocating) wikipedia , lookup
Basal metabolic rate wikipedia , lookup
Photosynthesis wikipedia , lookup
Evolution of metal ions in biological systems wikipedia , lookup
Microbial metabolism wikipedia , lookup
Adenosine triphosphate wikipedia , lookup
Electron transport chain wikipedia , lookup
Citric acid cycle wikipedia , lookup
Biochemistry wikipedia , lookup
Light-dependent reactions wikipedia , lookup
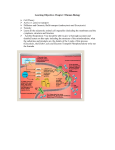
Vital Harvest Deriving Energy from Food Energy Currency and Energy Carrier Molecules • Energy harvesting—Everest’s deadly problem: having only 30 percent of the oxygen that would be available in the atmosphere at sea level. How many people know why we need to breathe oxygen? To extract energy from food (covered in this lecture). Does all energy harvesting require oxygen? • Relevant questions to be answered. – How do cells convert food into energy? Do they convert fats differently from carbohydrates? Do you get fat by eating fat or calories? – Why do we need to breathe? How does exercise affect metabolism? – What about energy supplements? What does it mean to have a fast or slow metabolism? Energy Currency and Energy Carrier Molecules • ATP is the most important energy storage molecule. – Potential energy from food breakdown is used to drive the endergonic synthesis of ATP (like recharging a battery). – The charged ATP has energy that can be released at any time (by breaking off the third phosphate to do a variety of actions). Energy Currency and Energy Carrier Molecules • Electrons from food carry energy to make ATP. – Electrons from glucose run downhill. Transferred by carriers, the electron drop powers uphill synthesis of ATP. – Electron transfers to molecules—redox reactions occur side by side. • One molecule is oxidized—loses electrons. • Another molecule is reduced—gains those electrons (reduces charge). Energy Currency and Energy Carrier Molecules • Intermediate electron carriers serve to shuttle electrons through these reactions, transferring energy as they go. – NAD+ (empty city cab) in redox reaction is an oxidizing agent (removes electrons causing a substance to be oxidized); it accepts a hydrogen atom and one electron becomes NADH (full cab). – NADH can carry electrons (proceed down energy hill) on to another acceptor, thus being regenerated (empty cab). – NAD+ is made by cells from the vitamin niacin • Enzymes coordinate all these transfers by bringing together the glucose derivatives with energy carrier molecules. Cellular Respiration • Overview of the three stages. – General reaction. • C6H12O6 +6O2 + ADP → 6CO2 + 6H2O + ATP – Energy coupling—downhill breakdown of glucose releases electrons, carried along and used to transfer energy to drive uphill synthesis of ATP. Cellular Respiration • Overview of the three stages. – Glycolysis—for eukaryotes, this is the first stage. It begins breakdown of glucose, yielding little energy, but it does transfer electrons to NAD+. On the plus side, it doesn’t require oxygen and occurs in the cytoplasm, and some prokaryotes and single-celled eukaryotes have long used it as the sole source of energy. – Krebs cycle and electron transport chain—evolved later, but generate larger quantities of energy; only problem is they occur only in mitochondria (only eukaryotes) and the electron transport chain requires oxygen. Glycolysis • Steps in the process. – Sugar in bloodstream enters cytoplasm, where this breakdown begins. Enzymes catalyze each reaction in metabolic pathway (first is hexokinase, which adds a phosphate from ATP called phosphorylation). – Although breaking sugar apart generates energy, it requires some activation energy (another ATP is used to attach another phosphate: –2 ATP total). Glycolysis • Steps in the process. – Rearrangement eventually leads to splitting the molecule in half from one 6-carbon sugar into two 3-carbon sugars (pyruvic acid is end product). – Oxidation by 2 NAD+ transfers electrons, and leads to the attachment of a high-energy phosphate to each sugar. Enough energy is generated by the eventual release of these four total phosphates in the next two steps to attach them to ADP to make ATP (+ 4 ATP). Glycolysis • Ledger. – Plus side—Very fast reactions cut glucose in half, generating small amount of energy (net 2 ATP), and electrons (2 NADH), but no oxygen was required. – Minus side—What is the next redox reaction where electrons can be transferred to empty the cab (NADH) for more passengers (NAD+)? Not much ATP made for all the work. Essay: When Energy Harvesting Ends at Glycolysis • Bacteria and certain eukaryotes can use only glycolysis. Problem—How can they recycle the NAD+? • Solution—alcoholic fermentation, yeast in absence of oxygen (bread and wine) must regenerate NAD+, so they dump electrons from NADH onto the acetaldehyde (converted from pyruvic acid and spewing off CO2), reducing it to ethanol, but regenerating the NAD+. • Solution—lactate fermentation, in animals in absence of oxygen (muscle fatigue), pyruvate accepts electrons from NADH and regenerates NAD+, but is converted into lactic acid (muscle burn). Essay: Energy and Exercise • Huge quantities of ATP are required to contract skeletal muscle, but ATP is generated in different ways depending on circumstances. • First burst of activity (6 seconds): cells have stockpile of ATP and phosphocreatine. • What is greatest source of energy at 30 seconds? Aerobic or anaerobic? What about at 10 minutes? Krebs Cycle • Sugar derivatives are oxidized to yield electrons in interior of inner membrane of mitochondria. The Krebs Cycle The Krebs Cycle • Steps in the process. – Acetyl CoA combines with oxaloacetic acid to make citric acid, continues around through a series of reactions that finally yield oxaloacetic acid again (cycle). – During the cycle of reactions, as the acetyl CoA is transformed, it is being oxidized by electron carrier molecules NAD+ and FAD. – Also, ATP and CO2 are produced. The Krebs Cycle • Ledger—6 NADH, 2 FADH2, and 2 ATP; acetyl CoA completely broken down into CO2. • Majority of electrons for next stage (electron transport chain). Electron Transport Chain (ETC) • Series of molecules in the mitochondrial inner membrane that are the destination of the electrons carried by NADH and FAHD2. • Steps in the process. – NADH arrives, and it bumps the ETC’s first carrier, which accepts the electrons, then passes them on along the chain (like a hot potato). – Movement of electrons at each transfer releases enough energy to power the movement of H+ ions from the inner compartment into the outer compartment (like heat of a hot potato dissipating as it is passed). The ions are being pumped against their concentration gradient (uphill). The Electron Transport Chain (ETC) • Steps in the process. – Hydrogen ions are allowed to flow downhill through an enzyme in the membrane called ATP synthase, like a water wheel spinning; as the ions pass, energy is used to transfer phosphate onto ADP to make ATP. • Greatest amount of ATP is made in this stage (32 ATP per glucose). • At the end of the ETC , which carrier accepts the electron? – 1/2 O2 + 2 electrons + 2 H+ = H2O Other Foods, Other Respiratory Pathways • Fats, proteins, and other sugars can also enter pathway to be converted to energy, but not in exactly the same way. • Food eaten in excess of caloric demands can also be converted from amino acids, fatty acids, and sugars into proteins, fats, and carbohydrates for structure or storage (98 percent of energy reserves of animals are fats). Other Foods, Other Respiratory Pathways • Example: Fats. – Triglyceride is converted to fatty acids and glycerol. – Glycerol is converted to glyceraldehyde phosphate (glycolysis intermediary) downstream. – Fatty acids can be broken apart and used to make acetyl CoA.