* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Osmosis
Survey
Document related concepts
Transcript
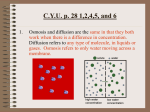
Name: _____________________________ Osmosis Read, highlight and then answer the questions. Diffusion of Water What does diffusion have to do with cells? Well, think of your blood as being made mostly of water (now I remembered to include it). This means that your cells are floating in a pool of mostly water (even though it’s red water ). Whichever has a higher concentration of water (inside the cell or outside the cell) determines if your cells need a drink or need to go to the bathroom. If your blood has more water particles then your cells, your cells pull water in. If your cells have more water particles in them than there is in your blood, then your cells release water. 1. If there is more water in the cell what will happen to the water? Will water go into the cell (draw an arrow pointing in) or will water move out of the cell (draw an arrow pointing out)? 2. Which has the higher concentration of water, outside your cell or inside your cell? Hopefully you drew the arrow coming out of the cell since there is more water inside the cell. So when your cells have more water than your blood, the cells push the water out and into your blood stream. This eventually leads to you going to the bathroom. It also works the opposite way. When you are playing outside on a hot summer day, your cells start screaming for water. 3. So where is the most water in this scenario? In your blood or in your cells? 4. Which has the higher concentration of water, outside you cell or inside your cell? Hopefully, you said in your blood. So when you are thirsty, your cells are sending signals to your brain asking for more water. This leads to you getting a drink. Osmosis We stated on previous night’s HW that diffusion is the movement of particles from an area of high concentration to an area of low concentration. There is a special case of diffusion which our cells depend on, the diffusion of water. This special case also has a special name, osmosis. So how does osmosis happen in the cell? Just like how perfume diffuses across the room spreading the smell out, water moves around to balance how much water is inside the cell and outside the cell. In the end, there will be an equal amount of water outside and inside the cell. Imagine osmosis (and diffusion) like a giant teeter-totter. The goal is to balance the teeter-totter. If there is too much water on one side of the cell (the large circle), the water would have to move across the cell membrane in order to rebalance the teeter-totter. Usually, your blood keeps a nice balance of water to keep the inside of the cell in balance. So what if your blood turned into pure water? What would happen to your blood cells? In this case, the water would flood into the cell since there’s a higher concentration of water outside your cells. This would cause the cell to fill up like a balloon and burst, not just one of them, but all of them. An opposite thought to pure water is drinking ocean water. If you’ve ever swam in the ocean, you were probably told not to drink the water. Why? Hopefully, you know that sea water has lots of salt. If there is more salt in the water and you drink it, suddenly you have less water molecules in your blood (outside your cells). Your cells will then have more water molecules inside them then outside them. Guess what? Your cells get tricked and start moving water outside of them. This is a problem because the water moves out causing your cells to shrivel up. This is also very similar to what is happening inside your body when you dehydrate. 5. What is Osmosis? Diffusion and your Cell There are also other types of particles moving back and forth across the cell membrane. Oxygen, for example, moves into and out of your cell. It’s the whole reason why you breathe. When you inhale, oxygen fills your blood. There is a higher concentration of oxygen in your blood which then moves to your cells. The higher concentration of oxygen moves from your blood into your cells just like water during osmosis. So why isn’t the diffusion of oxygen given a special name like water? It just wasn’t Sugar also moves back and forth across the membrane. When you first eat, your body fills your blood with a special sugar called glucose. The cell pulls glucose inside just like oxygen and water. When you take vitamins, your blood has a higher concentration of the minerals in the vitamin. Diffusion happens allowing the minerals to move across the membrane into the cells. What’s really cool though is that this movement happens without energy. I hate to say it just happens, but it does. The movement comes down to just diffusion (or osmosis), going from higher concentrations in one area to lower concentrations in another area. 6. When the high concentration of oxygen in your blood moves to the low concentration of oxygen in your cells, what is this called?