* Your assessment is very important for improving the workof artificial intelligence, which forms the content of this project
Download 1 Chemistry 3720 Exam 2 Spring 2001 This exam is worth 100
Survey
Document related concepts
Asymmetric induction wikipedia , lookup
Enantioselective synthesis wikipedia , lookup
Wolff rearrangement wikipedia , lookup
Bottromycin wikipedia , lookup
Stille reaction wikipedia , lookup
Baylis–Hillman reaction wikipedia , lookup
Wolff–Kishner reduction wikipedia , lookup
Elias James Corey wikipedia , lookup
Discodermolide wikipedia , lookup
Tiffeneau–Demjanov rearrangement wikipedia , lookup
Vinylcyclopropane rearrangement wikipedia , lookup
George S. Hammond wikipedia , lookup
Ring-closing metathesis wikipedia , lookup
Hydroformylation wikipedia , lookup
Transcript
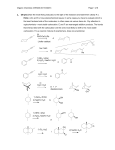
Chemistry 3720 Exam 2 Spring 2001 This exam is worth 100 points out of a total of 600 points for Chemistry 3720/3720L. You have 50 minutes to complete the exam and you may use the spectroscopy data sheet as needed. Good Luck. 1. (15 pts) Give the major products from each step in the following synthetic sequences. 1. Na2Cr2O7, H2SO4 H2O a. OH 2. CH3CH2NH2 catalytic H b. + 1. NaH, THF OH 2. O 3. H3O + 1. PDC, CH2Cl2 2. Ph3P=CH2 c. OH 3. H2SO4, H2O d. 1. H2SO4, ∆ OH 2. xs HI, ∆ 1. Na2Cr2O7, H2SO4 H2O e. OH 2. xs CH3OH, H + 1 2. (10 pts) Provide a detailed mechanism for the following reaction. O O xs H2O O H+ (catalyst) 3. (10 pts) Provide a detailed mechanism for the following reaction sequence to produce the alkene shown. O OH 1. Na2Cr2O7, H2SO4, H2O 2. m-CPBA, CH2Cl2 2 O 4. (15 pts) Provide the major organic products from each step in the following sequences. a. 1. OsO4, t-BuOOH, NaOH 2. NaIO4 b. O 1. 2 CH3MgBr O 2. H3O+ c. 1. m-CPBA 2. LiAlH4, THF O 3. H3O+ d. 1. m-CPBA, CH2Cl2 2. CH3MgBr, THF 3. H3O+ e. 1. PDC, CH2Cl2 OH 2. xs CH3OH, H+ 3 5. (10 pts) Design an efficient synthesis of the following product using 1-butanol as the only source of carbon. You may use any of the reactions and reagents used so far in 3719 or 3720. Show a complete retrosynthetic analysis of the problem, then the synthetic steps required to produce the compound in the forward direction. Note: There is more than one correct way of solving this problem. O 6. (15 pts) Provide the missing products in the following roadmap. H2SO4, H2O Na2Cr2O7 H2SO4, H2O IR : 3300 cm-1 1. NaH, THF 2. CH3CH2Br m-CPBA CH2Cl2 xs HBr, ∆ 13 2 products 4 C : 175 ppm 7. (5 pts) What product would you expect to be major when cyclopentyl methyl ketone is treated with m-CPBA in CH2Cl2? 8. (10 pts) Design a synthesis of the product shown using cyclohexene and methanol as the only organic starting materials. You only need to show a retrosynthesis if it helps you plan your synthetic scheme. O CH3 5 9. (10 pts) Provide the organic products from each step in the following reaction sequence. OH 1. Na2Cr2O7, H2SO4, H2O 2. m-CPBA, CH2Cl2 3. 2 CH3MgBr, THF + 4. H3O 6