* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Hemglobinopathies
Fetal origins hypothesis wikipedia , lookup
X-inactivation wikipedia , lookup
Frameshift mutation wikipedia , lookup
Gene therapy wikipedia , lookup
Oncogenomics wikipedia , lookup
Epigenetics of human development wikipedia , lookup
Public health genomics wikipedia , lookup
Polycomb Group Proteins and Cancer wikipedia , lookup
Site-specific recombinase technology wikipedia , lookup
Gene therapy of the human retina wikipedia , lookup
Gene expression profiling wikipedia , lookup
Microevolution wikipedia , lookup
Artificial gene synthesis wikipedia , lookup
Neuronal ceroid lipofuscinosis wikipedia , lookup
Designer baby wikipedia , lookup
Epigenetics of neurodegenerative diseases wikipedia , lookup
Genome (book) wikipedia , lookup
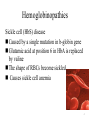
Hemoglobinopathies Dr. Shumaila Asim Lecture #6 1 Hemoglobinopathies • Disorders of hemoglobin caused by: – Synthesis of structurally abnormal Hb – Synthesis of insufficient quantities of normal Hb – Combination of both 2 HEMOGLOBINOPATHIES 1. Sickle cell anemia (Hb S) 2. Hemoglobin C disease (Hb C) 3. Hemoglobin SC disease (Hb S+ Hb C) 4. Thalasemia 3 Hemoglobinopathies Sickle cell (HbS) disease Caused by a single mutation in b-globin gene Glutamic acid at position 6 in HbA is replaced by valine The shape of RBCs become sickled Causes sickle cell anemia 4 5 Hemoglobinopathies Hemoglobin C disease: Caused by a single mutation in β-globin gene Glutamic acid at position 6 in HbA is replaced by lysine Causes a mild form of hemolytic anemia 6 Hemoglobinopathies Methemoglobinemia: • Caused by oxidation of Hb to ferric (Fe3+) state • Methemoglobin cannot bind oxygen • Caused by certain drugs, reactive oxygen species and NADH-cytochrome b5 reductase deficiency • Chocolate cyanosis: brownish-blue color of the skin and blood 7 Hemoglobinopathies Thalassemia: • Genetic blood disorder resulting in a mutation or deletion of the genes that control globin production. • Normal hemoglobin is composed of 2 alpha and 2 beta globins • Mutations in a given globin gene can cause a decrease in production of that globin, resulting in deficiency • aggregates become oxidized damage the cell membrane, leading either to hemolysis, ineffective erythropoiesis, or both. • 2 types of thalassemia: alpha and beta. 8 Demographics • The thalassemia gene may be maintained in the human population, in part because of the greater immunity of heterozygous individuals against malaria and is found in parts of the world where malaria is common • These include Southeast Asia, China, India, Africa, and parts of the Mediterranean. 9 Alpha Thalassemia • mutation of 1 or more of the 4 alpha globin genes on chromosome 16 • severity of disease depends on number of genes affected • results in an excess of beta globins 10 Silent Carriers (heterozygotes +/-) • 3 functional alpha globin genes • No symptoms, but thalassemia could potentially appear in offspring 11 Alpha Thalassemia Trait • 2 functional globin genes • results in smaller blood cells that are lighter in colour • no serious symptoms, except slight anemia 12 Hemoglobin H Disease • Second most severe form alpha thalassemia. • Usually caused by presence of only one gene producing alpha chains (--/-a). • Results in accumulation of excess unpaired gamma or beta chains. Born with 10-40% Bart's hemoglobin (γ4). Gradually replaced with Hemoglobin H (β4). In adult, have about 30-50% Hb H. γ4 β4 13 Hemoglobin H Disease • Live normal life; however, infections, pregnancy, exposure to oxidative drugs may trigger hemolytic crisis. • RBCs are microcytic, hypochromic with marked poikilocytosis. Numerous target cells. • Hb H vulnerable to oxidation. Gradually precipitate in vivo to form Heinz-like bodies of denatured hemoglobin. Cells been described has having "golf ball" appearance, especially when stained with brilliant cresyl blue. 14 Alpha Thalassemia Major • no functional globin genes • death before birth (embryonic lethality) 15 Bart’s Hydrops Fetalis Syndrome • Most severe form. Incompatible with life. Have no functioning alpha chain genes (--/--). • Baby born with hydrops fetalis, which is edema and ascites caused by accumulation serous fluid in fetal tissues as result of severe anemia. Also see hepatosplenomegaly and cardiomegaly. • Predominant hemoglobin is Hemoglobin Bart, along with Hemoglobin Portland and traces of Hemoglobin H. • Hemoglobin Bart's has high oxygen affinity so cannot carry oxygen to tissues. Fetus dies in utero or shortly after birth. At birth, see severe hypochromic, microcytic anemia with numerous NRBCs. • Pregnancies dangerous to mother. Increased risk of toxemia and severe postpartum hemorrhage. 16 Beta Thalassemia • mutations on chromosome 11 • hundreds of mutations possible in the beta globin gene, therefore beta thalassemia is more diverse • results in excess of alpha globins 17 Beta Thalassemia Trait • slight lack of beta globin • smaller red blood cells that are lighter in colour due to lack of hemoglobin • no major symptoms except slight anemia 18 Beta Thalassemia Intermedia • lack of beta globin is more significant • bony deformities due to bone marrow trying to make more blood cells to replace defective ones • causes late development, exercise intolerance, and high levels of iron in blood due to reabsorption in the GI tract • if unable to maintain hemoglobin levels between 6 gm/dl – 7 gm/dl, transfusion or splenectomy is recommended 19 Beta Thalassemia Major • • • • complete absence of beta globin enlarged spleen, lightly coloured blood cells severe anemia chronic transfusions required, in conjunction with chelation therapy to reduce iron (desferoxamine) 20 Myoglobin A globular hemeprotein in heart and muscle Stores and supplies oxygen to the heart and muscle only Contains a single polypeptide chain forming a single subunit with eight a-helix structures The interior of the subunit is composed of nonpolar amino acids 21 Structure of myoglobin 22 Myoglobin • The charged amino acids are located on the surface • The heme group is present at the center of the molecule • Myoglobin gives red color to skeletal muscles • Supplies oxygen during aerobic exercise 23 Myoglobin in disease • Myoglobinuria: Myoglobin is excreted in urine due to muscle damage (rhabdomyolysis) • May cause acute renal failure • Specific marker for muscle injury • Less specific marker for heart attack 24