* Your assessment is very important for improving the work of artificial intelligence, which forms the content of this project
Download Cardiovascular Physiology 2016
Intracranial pressure wikipedia , lookup
Action potential wikipedia , lookup
Threshold potential wikipedia , lookup
End-plate potential wikipedia , lookup
Homeostasis wikipedia , lookup
Electrophysiology wikipedia , lookup
Common raven physiology wikipedia , lookup
Hemodynamics wikipedia , lookup
Stimulus (physiology) wikipedia , lookup
Biofluid dynamics wikipedia , lookup
Haemodynamic response wikipedia , lookup
Circulatory system wikipedia , lookup
Defibrillation wikipedia , lookup
Electrocardiography wikipedia , lookup
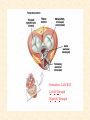
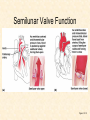
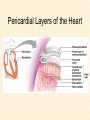
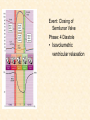
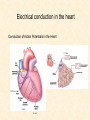
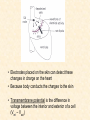
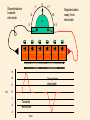
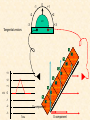
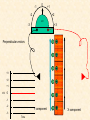
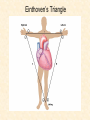
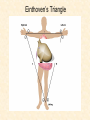
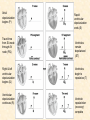
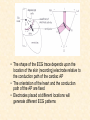
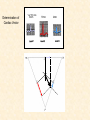
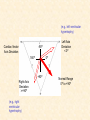
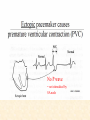
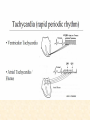
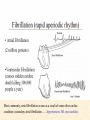
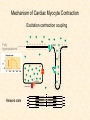
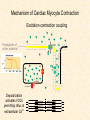
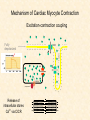
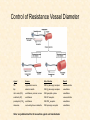
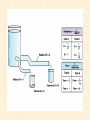
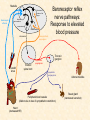
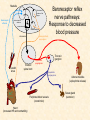
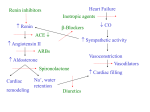
Cardiovascular Physiology 2016 Alan R. Burns, PhD University of Houston College of Optometry Room 2168 [email protected] 1 Organization of the Cardiovascular System 1. Heart composed of two pumps • Right ventricle sends blood to the lungs (pulmonary) while left ventricle sends blood to the body (systemic) 2. Organs perfused in parallel • allows organ-specific control of flow by regulating the diameter of arteries supplying a particular organ 3. Chambers of the heart perfused in series • • Rt Ventricle → Lft Ventricle → Rt Ventricle Output of the right and left ventricles must be matched or equal 4. CV system designed to maintain constant blood flow to brain and heart Blood Flow Through the Heart Function of the Ventricles • Ventricles generate pressure gradient, which produces blood flow • Cardiac output is the amount of blood pumped by a ventricle in a period of time • Cardiac output of right and left ventricles must be equal since the pulmonary and systemic circulations are in series • Two factors controlling cardiac output: CO = HR X SV Purpose of the Cardiovascular System Purpose of CV system is to maintain adequate blood pressure (BP) to achieve perfusion of the organs • Pumping action of the heart maintains flow or cardiac output (CO) • Diameter of arterial vasculature maintains resistance (R) • Heart and vasculature work together to control blood pressure, BP ~ CO x R Remember: LAB RAT Left AV Bicuspid Right AV Tricuspid Atrioventricular Valve Function Figure 18.9 Semilunar Valve Function Figure 18.10 Pericardial Layers of the Heart Things to remember about the Pericardium • Parietal layer - lines the internal surface of the fibrous pericardium • Visceral layer (epicardium) - lines the external surface of the heart • Double-walled sac - pericardial space is a fluid-filled cavity What else should you remember? • The pericardium: – Protects and anchors the heart – Prevents overfilling of the heart with blood – Allows the heart to work in a relatively friction-free environment Mechanical Events in Cardiac Cycle 4 Phases of the Cardiac Cycle 1. Inflow Phase: inlet valve open & outlet valve closed 2. Isovolumetric Contraction: both valves closed, no blood flow 3. Outflow Phase: Outlet valve open & inlet valve closed 4. Isovolumetric Relaxation: both valves closed, no flow Blood Pressure (mm Hg) Milliliters of Blood Event: Opening of AV Valves Phase: 1 Diastole • Rapid ventricular filling • Decreased ventricular filling (Diastasis) • Atrial contraction (atrial kick) Event: Closing of AV Valves Phase: 2 Systole • Isovolumetric ventricular contraction Event: Opening of Semilunar Valve Phase: 3 Systole • Rapid ventricular ejection • Decreased ventricular ejection Event: Closing of Semilunar Valve Phase: 4 Diastole • Isovolumetric ventricular relaxation Heart Sounds-Cardiac Cycle First Heart Sound (S1) • • • • “lub” closure of AV valves separated by ~ 10 msec M1 and T1 components Second Heart Sound (S2) • “dup” • closure of semilunar (aortic and pulmonary) valves • A2 and P2 components • “normal” S2 splitting occurs during inspiration (changes pleural pressure and blood flow)… P2 remains open longer S3 and S4 audible in children and young adults, but in the elderly: •S3 - congestive heart failure (vibration of ventricle walls and valves, resulting from early Phase 1 rapid ventricular filling) Systole •S4 - hypertrophic ventricle (vibration of ventricle walls and valves, resulting from late fromPhase Figure 121-2 slow ventricular filling) M1 T1 A2 P2 The Pressure-Volume Relationship The Pressure-Volume Loop Systole Diastole Starling’s Law of the Heart “…the mechanical energy set free in the passage from the resting to active state is a function of the length of the fiber.” In other words: The initial length of the myocardial fibers (end-diastolic volume) determines the amount of tension (pressure) generated during activation. Ernest Starling 1866-1927 Matching Cardiac Inflow and Outflow 1. Starling’s Law of the Heart is based on the LengthForce relationship 2. Increased blood return → increased EDV → increased sarcomere length 3. Increased sarcomere length → increased force by mechanisms that may include greater Ca2+ release from the SR and increased Ca2+-sensitivity of the contractile proteins Optimal A Term to be Familiar With Preload in the cardiac myocyte -- the initial sarcomere stretch before active tension development in the whole heart -- end-diastolic volume (EDV) correlates with the sarcomere length just prior to contraction Examples of Increased venous return (preload): • Exercise (increased skeletal muscle contraction squeezes veins) • Sympathetic activity constricts veins (capacitance vessels) and thereby decreases venous compliance • Increased rate and depth of respiration. During inspiration, lung expansion increases pulmonary blood volume; during expiration, lung deflation increases blood flow to left atrium Cardiac Contractility is a Measure of the Heart’s Intrinsic Contractile Performance Cardiac contractility is a measure the heart’s intrinsic ability to generate force (pressure) for a given fiber length (preload) Contractility is determined by a number of variables Contractility is approximated by several means such as dP/dt, ejection velocity, and ESPVR (End Systolic Pressure Volume Relationship) * Norepinephrine is an inotrope that increases contractility The Pressure-Volume Loop response to changes in contractility, preload, and after-load Cardiac Output The volume of blood ejected by the ventricle with each beat is called the Stroke Volume (SV) SV equals the difference between the End Diastolic Volume & the End Systolic Volume Cardiac output is SV multiplied by the Heart Rate (HR) CO = SV X HR CO = (EDV - ESV) X HR CO = (70 mL/ beat) X (70 beats/min) CO = 4,900 mL/min Factors Controlling Cardiac Output CO = HR x SV BP ~ CO x R 1. Heart rate: • Increasing HR → increased CO → increased BP 2. Stroke volume: • Increasing SV → increased CO → increased BP Cardiovascular Function at the Cellular Level Electrical conduction in the heart action potential is a transient alteration of the transmembrane voltage (membrane potential) across an excitable membrane in an excitable cell (e.g., myocyte) generated by the activity of voltage-gated ion channels embedded in the membrane action potential originates in “pacemaker” cells - autorhythmic action potential propagated through the heart via rapid and slow conduction pathways Excitation-contraction (EC) coupling mechanism by which the action potential promotes contraction in cardiac myocytes Modulation: sympathetic and parasympathetic Electrical conduction in the heart Conduction of Action Potentials in the Heart 0.5 m/s 0.05 m/s 2 m/s 4 m/s Electrical Conduction in the Heart Diversity of Action Potentials Membrane Potential (mV) 1 sec SA nodal cell myocardial contractile myocyte skeletal muscle myocyte 0 V cell -60 Ionic Basis of Resting Membrane Potential and Action Potential Pacemaker cell (i.e. SA nodal cell) close, Figure 14-16 Note: If current is carried by cyclic nucleotide-gated ion channels. These channels conduct Na+ and K+ and are thus described as non-selective cation channels. SA action potential – Ca++ and K+ Spontaneous depolarization – Na+ If currents and changes in K+ and Ca++ Ionic Basis of Resting Membrane Potential and Action Potential Contractile myocyte K+ - brake Na+ - gas Ca++ - slow gas (modulates) Figure 14-14 • No action potential – Outside of cells positively charged relative to inside – Outside of heart is positively charged • As AP passes over the heart, outside becomes negatively charged while inside becomes more positive (depolarization) • As repolarization passes over, outside becomes positively charged again • Electrodes placed on the skin can detect these changes in charge on the heart • Because body conducts the charges to the skin • Transmembrane potential is the difference in voltage between the interior and exterior of a cell (Vint – Vext) Depolarization wave front towards recording electrode 0 -1 +1 +2 -2 mV -3 (-) - +3 - - - - - (+) Electrode facing an approaching wave of depolarization records a positive potential Electrical current is greatest when the ratio of polarized to non-polarized tissue is 1:1; current decreases when <1:1 Depolarization wave front away from recording electrode 0 -1 +1 +2 -2 mV -3 (-) - +3 - - - - - (+) Electrode facing a receding wave of depolarization records a negative potential 0 -1 Depolarization towards electrode +1 Depolarization away from electrode +2 -2 mV -3 - +3 - - - - +3 +2 Away from electrode +1 mV 0 -1 -2 Towards electrode -3 Time - -1 0 +1 +2 -2 mV -3 +3 Tangential vectors +3 +2 +1 mV 0 -1 -2 Y component -3 Time X component -1 0 +1 +2 -2 mV -3 +3 Perpendicular vectors +3 +2 +1 mV 0 -1 -2 Y component -3 Time X component Willum Einthoven 18601927 Nobel Prize 1924 Einthoven’s Triangle Einthoven’s Triangle ST-segment = plateau phase of ventricular action potential QT-interval = duration of systole * Atrial repolarization is masked by QRS wave Atrial depolarization begins (P) Travel time from SA node through AV node (PQ) Right & left ventricular depolarization begins (Q) Ventricular depolarization continues (R) Rapid ventricular depolarization ends (S) Ventricles remain depolarized (ST) Ventricles begin to repolarize (T) Ventricle repolariztion (recovery) complete • The shape of the ECG trace depends upon the location of the skin (recording) electrode relative to the conduction path of the cardiac AP • The orientation of the heart and the conduction path of the AP are fixed • Electrodes placed at different locations will generate different ECG patterns • The potentials recorded depend on the angle between the cardiac dipole and the leads on the body surface Precordial Leads (precordium = portion of body over the heart and lower chest (thorax)) *12 leads used in a standard electrocardiogram: the standard bipolar limb leads I–III, 3 augmented unipolar limb leads, and 6 precordial leads Determination of Cardiac Vector 6mm Lead 1 11mm Lead 2 4mm Lead 3 L (e.g., left ventricular hypertrophy) Cardiac Vector Axis Deviation Left Axis Deviation <0° -90° 180° 0° +90° Right Axis Deviation >+90° (e.g., right ventricular hypertrophy) L Normal Range 0° to +90° Causes of Arrhythmias SA node sets the rhythm • if SA node becomes damaged, AV node will take over but at a slower pace • if AV node becomes damaged, Bundle of His will take over but pace is too slow (28 bpm) and is not really compatible with life Ectopic Foci • abnormal conducting or contractile cells that generate AP’s and override the impulse rhythm of the SA or AV node No P wave - not stimulated by SA node Tachycardia example Abnormal Impulse Conduction • block of conduction leading to re-entry • block can occur as a result of myocarditis, cardiomyopathy, congentital defect, rheumatic fever, myocardial infarction, etc. Most commonly, atrial fibrillation occurs as a result of some other cardiac condition (secondary atrial fibrillation……hypertension, MI, myocarditis). Pacemaker – provides electrical stimulation for slow ventricular contraction Defibrillator - fires a preset number of rapid pulses in succession in an attempt to terminate ventricular tachycardia Electrical conduction in the heart Efficient Distribution of the Action Potential Throughout Cardiac Myocytes via “T-System” Transverse tubules (t-system) • invaginations of the sarcolemma • open to the extracellular space • carry propagated action potentials deep within myocardial cell Mechanism of Cardiac Myocyte Contraction Excitation-contraction coupling Ca Ca Ca Ca + + + + + + + + + + + + VOC - - - - - - - - - - - - Ca Ca Ca Ca Ca Ca SERCA ATP Ca Ca + + phospholamban + + + Ca - - - - - Ca Ca Ca Ca Ca - - Relaxed state - - - - - - - - - - - - - - - - - Ca RyR Ca Ca Ca + + + + + + + + + + + Ca + + + + + - - - - - Ca Ca Ca Ca Ca + + Fully hyperpolarized Ca Ca T-tubule Ca Ca Ca Mechanism of Cardiac Myocyte Contraction Excitation-contraction coupling Ca Ca Ca Ca - - - - - - + + + + + + + + + + + + - - - - - - Ca Ca Ca Ca SERCA ATP Ca Ca + + phospholamban + + + Ca - - Action potential - - - - - - - - - - - - - - - - - Ca Ca - - - - - Ca + + + Ca Ca Ca Ca Ca + + + + + + + + Ca Ca RyR Ca Ca + + + + + Ca Ca - - - - - Ca Ca Ca VOC + + Propagation of action potential Ca Ca T-tubule Ca Ca Ca Mechanism of Cardiac Myocyte Contraction Excitation-contraction coupling Ca Ca Ca Ca Ca Ca - - - - - - - - - - - - VOC + + + + + + + + + + + + Ca Ca Ca Ca Ca Ca SERCA + Ca - phospholamban Ca - ATP Ca - - - Ca + + + + Ca Ca Ca Ca Ca - - - - - - - - - - - - - + + Ca Ca RyR Ca Ca Ca + + + + + + + + + + Ca Ca - - - - - + + + + + Ca Ca Ca Ca + + Propagation of action potential Ca Ca T-tubule Ca Ca Ca + + + Depolarization activates VOCs permitting influx of extracellular Ca2+ Mechanism of Cardiac Myocyte Contraction Excitation-contraction coupling Ca Ca Ca Ca - - - - - - - - - - - - VOC + + + + + + + + + + + + Ca Ca Ca Ca Ca Ca Ca Ca Ca Ca Ca SERCA Ca ATP + Ca Ca - phospholamban - - - Ca Ca Ca + + + + Ca Ca RyR Ca Ca + + + + + + + + + + + + + + + + Ca Ca Ca Ca Ca - - - - - - - - - - - Ca Ca - - - - - + + + + + Ca Ca Ca Ca - - Fully depolarized Ca Ca T-tubule Ca Ca Ca + + + Release of intracellular stores Ca2+ via CICR Excitation-contraction coupling in contractile Cardiac Myocytes 1. Level of intracellular Ca2+ determines magnitude of contraction: • more Ca → bigger contraction → greater cardiac output → greater blood pressure 2. Ca2+ stored intracellularly in sarcoplasmic reticulum (SR) 3. When Ca2+ is released from SR it binds Troponin C which forces Tropomyosin off the myosin binding region of actin – promotes contraction 4. Reuptake of Ca2+ by SR required for cardiac muscle relaxation between beats 5. Ca2+ release and reuptake happens ~70 times/min at resting heart rate Modulation of Contractility by Cardiac Glycosides Cardiac glycosides inhibit Na+/K+-ATPase activity. X X Na+ Na+ Ca2+ Na+ Ca2+ Na+ Na+ Na+ Ca2+ Ca2+ cardiac glycosides (digitalis) Ca2+ Ca2+ Ca2+ Ca2+ Ca2+ Ca2+ Ca2+ Ca2+ Ca2+ Ca2+ Ca2+ Ca2+ Ca2+ Ca2+ Ca2+ How does inhibition of Na+/K+ATPase activity eventually affect cardiac contractility? Digitalis purpurea (Purple Foxglove) Modulation: Sympathetic and Parasympathetic Pathways Sympathetic Stimulation Parasympathetic Stimulation Catecholamines (epinephrine and Acetylcholine (ACh) acts via norepinephrine) act via β1muscarinic receptors adrenergic receptors Leads primarily to decreased Leads to 1) more forceful heart rate contractions and 2) increased heart rate Sympathetic stimulation leads to more forceful contraction P Ca2+ Ca2+ Ca2+ Ca2+ Ca2+ P Ca2+ Ca2+ Ca2+ Ca2+ P P P Sympathetic stimulation leads to increased heart rate Na+ (through non-selective cation channels) and Ca2+ (through VOCs) influx leads to a more positive start point (depolarization point) and more rapid depolarization ( rate of SA node firing) Figure 14-17 Na+ entry (If) nodal cells *Norepinephrine & epinephrine have chronotropic and inotropic effects on heart rate and contractility, respectively more rapid depolarization from more positive startpoint nodal cells Heart Rate Parasympathetic stimulation leads to decreased heart rate (little effect on force of contraction) Muscarinic receptor (M2) activation by ACh inhibits Adenylate Cyclase (AC), resulting in a fall in cAMP levels ACh M2 receptor promotes activation of Kchannels Lower cAMP levels decrease the activity of non-selective cation channels responsible for If current (-) P ATP PKA Lower cAMP levels decrease the activity of VOCs (T-type) PKA K+ efflux and Na+ and Ca2+ influx leads to hyperpolarized pacemaker potentials and slower depolarization The slower depolarization rate results in a longer time between action potentials and thus a lower HR *ACh is a chronotrope cAMP Figure 14-17 Special Role of Large Outflow Arteries The large outflow arteries of the heart are designed to absorb the energy from the ejected blood and release it more evenly over time in order to maintain a more constant blood pressure. 1. Contain many layers of smooth muscle • Necessary for vessel wall to withstand the high pressures 2. Contain multiple layers of elastic elements • Necessary to absorb and redistribute the force over time Vasculature • Arterioles, the smallest arterial subdivision, are the site of resistance control Control of Resistance Vessel Diameter Agent Source Site of Action Result NE sympathetic neurons VSM α1 adrenergic receptors vasoconstriction Epi adrenal medulla VSM β2 adrenergic receptors vasodilation nitric oxide (NO) endothelium, perivasc. nerves VSM guanylate cyclase vasodilation endothelin (ET) endothelium VSM ET receptors vasoconstriction prostacyclin (PGI2) endothelium VSM PGI2 receptors vasodilation adenosine surrounding tissue metabolite VSM purinergic receptors vasodilation Note: very abbreviated list of vasoactive agents and mechanisms Jean Louis Marie Poiseuille 1799-1869 Determinants of Mean Arterial Blood Pressure 1. Arteriolar Resistance • Resistance (R) ~ Lη/r4 • Vessel diameter is the primary determinant of vascular resistance • Vascular diameter controlled by local factors as well as the endothelium and sympathetic nerves slight constriction r = 0.8 mm slight dilation r = 1.0 mm r = 1.2 mm CSA = 2.01 mm2 CSA = 3.14 mm2 CSA = 4.52 mm2 R ~ 2.44 R ~ 0.48 R ~ 1.00 Determinants of Mean Arterial Blood Pressure 1. Arteriolar Resistance • Resistance (R) ~ Lη/r4 • Vessel diameter is the primary determinant of vascular resistance • Vascular diameter controlled by local factors and sympathetic nerves 2. Cardiac Output • • Blood Pressure (BP) ~ CO x R Increased CO → Increased BP 3. Total Blood Volume • Increased blood volume → BP 4. Distribution of blood • • Most blood is normally in “venous reserve” Contraction of veins makes more blood volume available Components of the Homeostatic System 1. Aortic and Carotid Baroreceptors • • Sense arterial blood pressure Send signals to the medullary cardiovascular control center 2. Medullary Cardiovascular Control Center • Responds to baroreceptors by modulating sympathetic and parasympathetic nerve activity 3. Sympathetic and Parasympathetic Nerve Activity • • Affect vascular resistance Affect CO Tonic stimulation SA node Medulla Baroreceptor reflex nerve pathways: Response to elevated blood pressure Dorsal motor nucleus of the vagus Nucleus tractus soltarius Baroreceptor afferents - vasomotor area Nucleus ambiguus interneuron Thoracic spinal cord Carotid sinus parasym postgang parasympathetic preganglionic sympathetic preganglionic Thoracic ganglion sympathetic postganglionic Peripheral blood vessels (dilation due to loss of sympathetic constriction) Heart (decreased HR) Adrenal medulla Sweat gland (decreased secretion) Medulla Baroreceptor reflex nerve pathways: Response to decreased blood pressure Dorsal motor nucleus of the vagus Nuclues tractus solitarius Baroreceptor afferents - vasomotor area Nucleus ambiguus interneuron Carotid sinus parasympathetic preganglionic Thoracic spinal cord parasym postgang Heart (increased HR and contractility) Thoracic ganglion sympathetic preganglionic sympathetic postganglionic Peripheral blood vessels (constriction) Adrenal medulla (epinephrine release) Sweat gland (secretion)